A Rare Case Report of Venous Thromboembolism in a Child
Thromboembolism, or the development of a clot within blood vessels, can occur in arteries or veins. Venous thromboembolism (VTE), a leading cause of adult morbidity and mortality, has a lower incidence in children than in adults and carries significant morbidity in both. The incidence of VTE in children is not precisely known because few prospective studies of this subject exist. We present through this case, a severe presentation of deep vein thrombosis (DVT), whose etiology is multifactorial.
Keywords: Venous thromboembolism (VTE); Deep Vein Thrombosis (DVT); Inferior Vena Cava (IVC); Pulmonary Embolism (PE); Transthoracic Echocardiography (TTE)
A study by Raffini, et al. found that the annual rate “of venous thromboembolism” grew from 34 to 58 cases per 10,000 hospital admissions between 2001 and 2007 [1-3]. The incidence of thromboembolism peaks in newborns and infants younger than one year then remains very low until adolescence, when the incidence begins to increase [4]. Overall, there is no racial nor sex predilection. Virchow’s triad (stasis, endothelial injury, and hypercoagulability) is the basic pathological process underlying VTE [5]. The etiologies could be inherited or acquired. Symptoms and signs of VTE are nonspecific, and a high index of suspicion is required for timely diagnosis, treatment and prevention of morbidity and mortality.
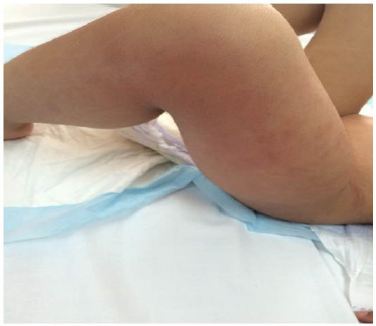
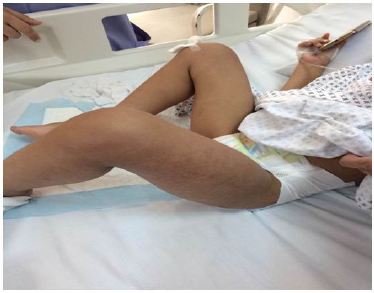
A Four year old male patient, known to have Neurofibromatosis type I, was transferred to our Hospital for fever, generalized maculopapular rash, and worsening clinical condition. History goes back to 2 days prior to presentation, when he started to have high grade fever reaching 40 degrees, partially relieved by antipyretics, associated with diffuse maculopapular non pruritic rash, mild episodic nonproductive cough, decreased oral intake and generalized fatigue. No abnormal movements, headache, nausea, vomiting, diarrhea, constipation, urinary symptoms, weight loss, exposure to sick contacts, or history of recent travel. Past medical history was positive for Neurofibromatosis type I, with thalamic lesions and subsequent partial seizures, controlled by Levetiracetam (Keppra), and evident neurodevelopmental delay. No past surgeries and vaccinations were up to date for age. Family history was negative for inherited diseases and no consanguinity. He was admitted to another Hospital where he was looking ill, irritable, tired; with diffuse non pruritic, blanching maculopapular rash, sunken eyes, and dry oral mucosa with erythematous tonsils. No cervical lymphadenopathy and no neck stiffness. Laboratory studies upon admission showed WBC 10300, Segmented 93%, Lymphocytes 2%, CRP 14.1 mg/dl which is considered high level as upper normal is 5, rapid Streptococcus test was positive, and Na 124 mEq/L, and CO2 19 mEq/L. He was diagnosed as having Streptococcal Tonsillitis associated with hyponatremic dehydration for which was started on IV hydration and Amoxicillin Clavulinate. After 6 hours, he developed left lower limb pain and refusal to walk. No history of trauma, no morning stiffness, no joint swelling. He was transferred to our service for further investigations and treatment. Upon presentation he was looking ill, irritable, left knee flexion, with very mild non pitting edema noticed, mild erythema and warm skin, also had diffuse tenderness on limb palpation, decrease active and passive movement with limitation of the range of motion of both hip and knee at the left side. Otherwise he had normal physical examination of the right lower limb, and positive pedal pulse bilaterally (Figure 1 and 2).
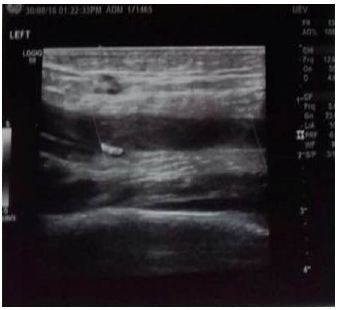
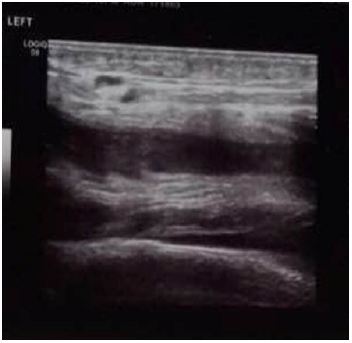
Our differential diagnosis was: Septic arthritis, deep vein thrombosis (DVT), psoas muscle abscess, transient synovitis, osteomyelitis and trauma. Laboratory tests revealed: Hb 11.2, WBC 9800, segmented 90%, lymphocytes 2%, platelets 150, Na 122mEq/L, and the rest of electrolytes were normal, D-Dimer 42230 ng/ml (high). As for the rest PT 51%, INR 1.49 (prolonged), with normal PTT, ESR, LFT’s, LDH, fibrinogen and CPK. Urine analysis and spot urine for electrolytes were normal. Color-Doppler ultrasound of lower limbs revealed left deep femoral vein thrombosis affecting its half superior part, with partial permeability of its half inferior part and left popliteal vein thrombosis (Figure 3 and 4) [6,7].
The remaining investigations such as bilateral hips, pelvis and left lower limb were normal. Ultrasound bilateral hips and knees also were normal. Ultrasound of abdomen and pelvis as for psoas muscle were normal. The patient was started on IV hydration, Amoxicillin IV, and started immediately on Enoxaparin (Lovenox) (1mg/kg/dose) twice a day. The Hematology-Oncology opinion suggested adjustment Enoxaparin dose according to anti factor Xa level and investigate complete coagulation and cardiovascular panel. The cardio-vascular team suggested another Color-Doppler ultrasound left lower limb reaching the iliac vessels to assess if any extension and revealed no extension and no need for inferior vena cava (IVC) filter. The remaining laboratory results revealed: Normal peripheral smear, Factor V Leiden, Factor V PCR, Factor II mutations, Factor XIII mutations, Antiphospholipid Ab, Anticardiolipin Ab (IgM and IgG ), B2GPI (IgM + IgG), Homocystein level, and Lipoprotein a levels. Antithrombin III activity was 53% with normal range 75-125. Protein C activity was 15% with normal range 70-140. Protein S activity was 29% with normal range 64-149. Cardiovascular panel was Heterozygote for B Fibrinogen (G/A). MTHFR gene was double Heterozygote: MTHFR C677T (C/T) and MTHFR A1298C (A/C) [8]. But, in one hand, Antithrombin III is consumed after DVT (It is an effect rather than a cause), in the other hand, protein C and S are Vitamin K dependent (Our patient had prolonged INR at presentation), and when we talk about deficiency, levels should be low near zero. So these levels must be repeated one to two months after successful treatment to decide on it finally. Now, B Fibrinogen mutation can cause DVT? A study done by Jolanta, et al. on 2012 showed that elevated fibrinogen levels are associated with risk of pulmonary embolism but not with DVT [9]. A study after which new hypothesis comes up, in that the mechanism of formation of DVT is different from that of pulmonary embolism! MTHFR mutation can cause DVT? In fact, MTHFR mutation causes elevated Homocystein level, which by its own is a well-known risk factor for DVT, but as mentioned above, our patient had normal Homocystein level. Finally, what can be the cause of that DVT? It must be multifactorial, including several factors joined together to cause it, let’s say the infection, mild dehydration, hypoactivity, and the multiple heterozygotes genetic defects.
Our patient was given IV hydration, 10 days course of Amoxicillin and Lovenox twice daily, his hyponatremia was corrected, he became afebrile after 3 days. He was switched to oral anticoagulant (Warfarin), and serial Doppler ultrasounds of left lower limb were done, showing gradual improvement until normal. He is planned to continue anticoagulation for 1 year, and then will repeat all the coagulation and cardio vascular panels.
We needed timeout to discuss the case while waiting the rest of the results, and we ended up with several points: A) - Our patient had NF type I, increase the risk of venous thromboembolism? In the review of literature, there was only one case of DVT in an adolescent with peripheral NF type I, but it was caused by an anatomical compression due to femoral exostosis [6]. B) - Furthermore, NF is a well-known cause of arterial and not venous stenosis (renal artery, cerebral arteries, carotid artery…) due to mutation in or deletion of the NF1 gene. The gene product neurofibromin serves as a tumor suppressor, and is expressed in blood vessel endothelial and smooth muscle cells [7,8]. C) - Our patient had Strep throat, increase the risk of VTE? In the review of literature, no DVT case was found to be caused by Streptococcus pharyngitis. D) - Again our patient was taking Kepra, does it causes VTE? By reviewing all its side effects, from the most common ones to the least common ones, DVT was not one of the mentioned side effects. Thromboembolism can occur in arteries or veins; it can be acquired or hereditary. In 1845, Virchow postulated that three factors were important in the development of thrombosis: flow stasis, endothelial damage, and hypercoagulable state. The physiology of hemostasis is remarkably complex and reflects a fine balance between an uninterrupted flow of blood (i.e., fluid) and a rapid, localized response to vascular injury (i.e., clotting) [5]. The process of hemostasis is traditionally divided into: A) - A cellular phase (primary hemostatic phase with platelets and the vascular wall) and B) - A fluid phase (secondary hemostatic phase with plasma proteins) [5]. The fluid phase is divided into the following 3 processes, abnormalities in any of which can contribute to hypercoagulable or hypocoagulable states: A) - The multiple-step zymogen pathway that leads to thrombin generation, B) - Thrombin-induced formation of a fibrin clot and C) - Complex fibrinolytic mechanisms aimed at limiting clot propagation [5,10] (Figure 5).
The etiologies can be divided into inherited and acquired disorders (Table 1 and 2):
The presentation of thromboembolism is widely variable, and depends upon its nature (venous, arterial…), location (cerebral, limbs, pulmonary…), depth (superficial, deep…), and patient’s age (neonate, infant, children…). As examples, DVT can present with severe acute pain, swelling, erythema and warm limb… Pulmonary embolism can present as acute chest pain, shortness of breath, petechial, tachycardia, diaphoresis…CNS thrombosis may present with vomiting, lethargy, seizure, limb weakness, headache…Renal vein thrombosis may present with flank pain, hematuria… And arterial thrombosis can present as acute pain, cold limb, decreased or absent pulse, numbness, pallor…Once a clot is suspected, the patient’s workup should include: CBCD, Peripheral smear, PT, PTT, Fibrinogen, and D-Dimer. Then first line workup for anticoagulation should be ordered, and includes: Activated protein C resistance and/or the factor V Leiden mutation, protein C, free and total protein S, Antithrombin, Lupus anticoagulant, Anticardiolipin antibodies, Prothrombin gene 20210A mutation, Lipoprotein a levels, Plasma Homocysteine values (which can be measured after fasting or at 4 h after a loading dose of methionine 100 mg/kg). There is several imaging modalities that aid for the diagnosis of thromboembolism: A) - Contrast venography: is reference standard for DVT but it is invasive, not reliable for jugular veins, has difficulty in canulating small veins and is contraindicated in patients allergic to contrast, B) - Color-Doppler ultrasound: is used as primary diagnostic tool. In vessels with thrombosis, Doppler signals are absent, and the lumen cannot be compressed with direct pressure, C) - Ventilation-perfusion scan: was used to diagnose Pulmonary edema (PE). A high-probability scan is one that shows a peripherally based perfusion defect with normal ventilation (mismatch), but it has largely been replaced with CT scan or MRA after having elevated D-Dimer. D) - MRI, MRA head: are the modalities of choice for evaluating a child with suspected CNS thrombosis. E) - Head CT with IV contrast: is sometimes useful for detecting sinus-venous thrombosis, but MRI and MRA are better than CT scanning for detecting early arterial ischemic stroke because CT findings are often normal. F) - CXR: Is more helpful for suggesting alternative diagnoses, such as pneumonia, than for diagnosing PE, but it can show non-specific findings such as a small pleural effusion and a wedge-shaped, pleural-based opacity of pulmonary infarction. G) – Transthoracic echocardiography (TTE): should be done when we are suspecting a cardiac cause of thromboembolism. After diagnosing VTE, patient should be admitted to floor or PICU depending on their respiratory and neurologic status, then\ anticoagulation should be begun with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH), followed by oral anticoagulation with warfarin. Children require daily follow-up until their (INR) is > 2 on 2 successive days. Patient’s INR should be monitored more closely than usual if changes occur in the patient’s medications or diet. If LMWH is used, should be obtained an anti– factor Xa level and adjust the dose to achieve a level of 0.5-1 U/mL [10]. The duration of therapy depends on the underlying problem [11]. A) - Children with mechanical heart valves or recurrent thromboembolism require anticoagulation indefinitely. B) - Children with thromboembolism and persistent risk factors may be treated for 3 months and then switched to low-dose warfarin until the risk factor is no longer present. C) - Uncomplicated DVT can be treated for 3-6 months. There is some special circumstances that we have to keep in mind: A) - Neonates have low levels of antithrombin and plasminogen, which cause relative resistance to heparin and thrombolytic agents, respectively [11]. B) - Many clotting factors are consumed in a clot, and a low factor level may be an effect rather than a cause of thrombosis; therefore, most clotting factors should be evaluated 1-2 months after successful treatment of the clot. C) - Surgical thrombectomy may be necessary, especially after major cardiac surgery or if thrombolytic agents fail or are contraindicated, or if a limb or organ are threatened. D) - Vitamin K directly interferes with the effectiveness of warfarin and potentially increases the risk for recurrent thrombosis, thus daily intake of foods high in vitamin K, such as green, leafy vegetables, should be kept at a consistent level [12]. E) - Children with thromboembolism are sometimes restricted to bed rest for the first 24-48 hours to decrease the risk of PE. However, this practice has never been shown to reduce the risk of embolization [13].
Venous thromboembolism is increasingly recognized in the pediatric population. Approximately two thirds of VTEs are associated with the use of central venous lines. Their clinical manifestations depend on the location and extension of the thrombus, thus a high index of suspicion is required for timely diagnosis, and treatment (medical vs. surgical) should be started as soon as possible, and then be directed depending on the etiology. Etiologies could be of inherited or acquired disorders or conditions, but sometimes not a single factor or condition can explain the situation, like our case mentioned above, where we tried to define it as multifactorial.
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.