Adult Alveolar Paranasal Rhabdomyosarcoma; A Rare Aggressive Disease with Pulmonary, Brain and Skeletal Metastasis: Review of an Institutional Experience
Rhabdomyosarcoma (RMS) is predominantly seen in children, most commonly occurring in the head and neck region while extremity tumors are more common in adults. An adult onset RMS of nasal cavity and paranasal sinus is of exceedingly rare occurrence accounting for only 1.5% of reported head and neck cases. Adult RMS has worse outcome and survival than children with similar disease. We hereby review a highly aggressive and fulminant case of adult alveolar RMS where a 49-year-old male presented with complaints of left nasal stuffiness and left neck swelling followed by swelling of left cheek and proptosis within two months duration. Radiological imaging scans showed soft tissue lesion involving left nasal cavity extending to left maxillary sinus with intra-orbital and anterior cranial fossa infiltration without any distant organ involvement. Immune-histopathology revealed alveolar-type RMS. He was treated with induction chemotherapy followed by external beam radiotherapy (EBRT). He underwent left maxillectomy and ipsilateral neck dissection but developed local-regional recurrence within three weeks of surgery. While on adjuvant chemotherapy he developed pulmonary, brain and spinal metastasis for which he was palliated with EBRT. However, his condition kept on deteriorating on second line chemotherapy and he finally succumbed to this fulminant illness. With a background of our case, we discuss this disease of paranasal sinuses in adults to highlight its extreme aggressiveness and fulminant behaviour, rarity, the diagnostic and therapeutic challenge it presents, the overall dismal prognosis of this malignancy once dissemination occurs and summarize the recent advances in treatment.
Keywords: Rhabdomyosarcoma; Adult; Alveolar; Paranasal sinuses; Head and neck; Metastasis
List of abbreviations: RMS: Rhabdomyosarcoma; STS: Soft tissue sarcoma; EBRT: External beam radiotherapy (EBRT); PNS: Paranasal sinus; RT: Radiotherapy; CT: Computed tomography; WB-PET: Whole body positron-emission-tomography; FDG: Fluoro-deoxy-glucose; H & E: Haematoxylene and eosin; IHC: Immunohistochemistry; TTF-1: Thyroid transcription factor-1; LCA: Leucoyte common antigen; NACT: Neo-adjuvant chemotherapy, MRND: Modified radical neck-dissection, CNS: Central nervous system, RMS NOS: RMS not otherwise specified; IRS: Intergroup Rhabdomyosarcoma Study; OS: Overall survival; DFS: Disease free survival; MFS: Metastasis-free survival; FFS: Failure-free-survival; EFS: Event-free survival; CWS: Cooperative weichteilsarkom studiengruppe; VEGFR: Vascular endothelial growth factor receptor, EGFR: Epidermal growth factor receptor; PDGFR-A: Platelet-derived growth factor receptor-A; mTOR: Mammalian target of rapamycin; Hh: Hedgehog; IGF: Insulin-like growth factor
RMS is a highly malignant STS which arises from undifferentiated mesoderm or myotome-derived skeletal muscle. RMS is the most common childhood STS, accounting for more than 50% of all cases. In contrast, adult RMS is extremely rare [1]. STS constitute less than 1% of all adult malignancies, and RMS accounts for 3% of all soft tissue sarcomas [2]. Adult RMS has poor prognosis compared to childhood RMS [3] with high propensity for local and distant dissemination to lymph-nodes, lung, skeletal system and brain [4-6]. Adult RMS shows very rapid evolution, diagnosed most often at an advanced stage [7]. Truncal and extremity tumors are more common in adults Adult alveolar RMS shows more propensities for trunk and extremity sites while PNS locations are very rare [8]. Due to paucity of large randomized clinical trials apart from few retrospective series, treatment of adult RMS has been extrapolated from paediatric cases. Adults RMS have been reported to have poor outcomes, although there is evidence that when treated aggressively using paediatric-type protocols, the prognosis may be similar to that of younger patients which is better [8]. Recent advances in research of certain molecular receptors, cytogenetics and biologic pathways that are involved in RMS have increased our understanding of the disease and discover novel targeted therapies in paediatric cases but still much less are known about the adult variants. Multi-agent chemotherapy and adequate dosage RT of the primary tumor is essential for maximizing the chance of cure [8].
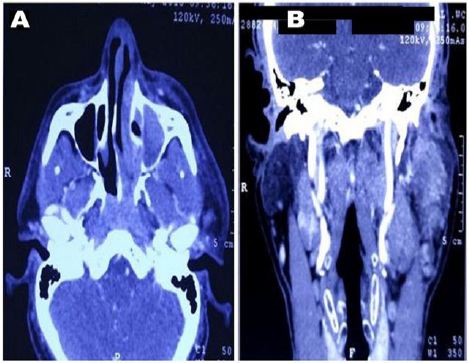
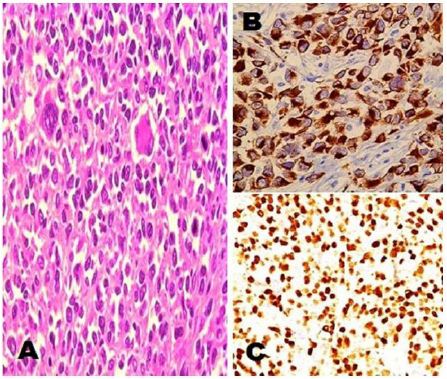
Data from malignant disease and treatment centre cancer registries of two apex tertiary care cancer institutes; Research and Referral hospital, New Delhi and Southern Command Hospital, Pune, India from January 2013 to November 2017 were taken. There were a total of 15600 head and neck cancer patients including paediatric and adults of both sexes of which 47 cases were of paediatric head and neck RMS of embryonal histology. Of the 47 cases, 46 cases were of orbital RMS and one child was with PNS involvement. Only one patient with adult alveolar RMS of PNS was encountered in these five years which is being summarized here. A 49-year-old male with no known comorbidities presented with history of left sided nasal stuffiness and neck swelling on same side. He took some over-the-counter antibiotics for upper respiratory tract infection with which he got no relief. Within few days he developed left sided facial swelling associated with proptosis. CT scan of head and neck showed a 5.6×5.9 cm lobulated soft tissue mass lesion epicentered in left nasal cavity extending into left maxillary sinus with intra-orbial and anterior cranial fossa infiltration and enlarged left cervical and supraclavicular lymph-nodes (Figure 1). WB-PET scan also showed FDG avid lesion left nasal and left maxillary sinus with similar extensions with no distant organ involvement. Trans-nasal biopsy was performed which showed abundant small blue cells with scattered rhabdomyoblasts. IHC stained positive for desmin and myogenin while it stained negative for p63, TTF-1, CD-99, LCA, S-100, and melan-A (Figure 2). He was diagnosed as a case of alveolar RMS of PNS.
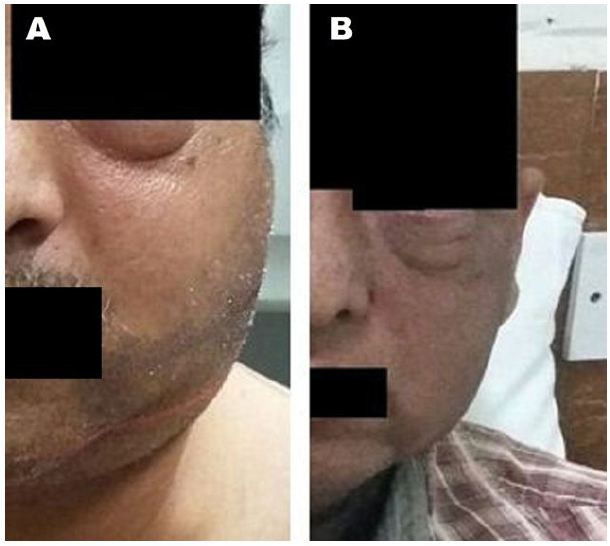
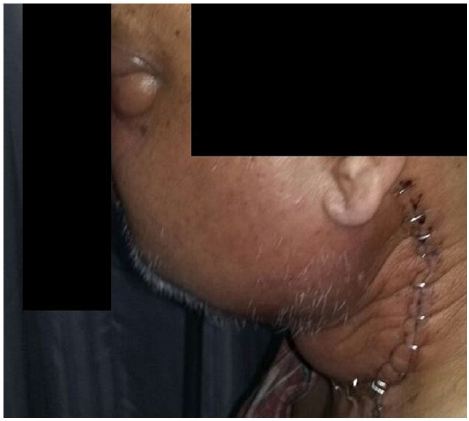
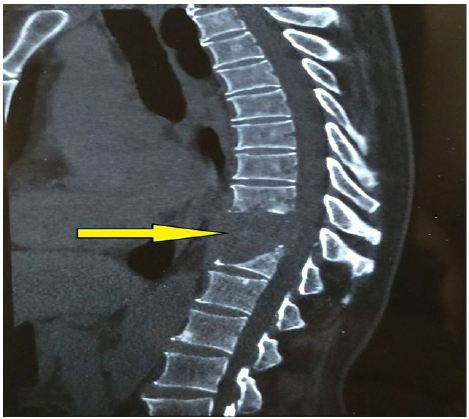
Patient was started on induction chemotherapy regimen with VAC (vincristine1.6 mg/m2, actinomycin-D 0.45 mg/kg and cyclophosphamide 1200 mg/m2) was given as intravenous bolus infusion on day 1, followed after 21 days by IE (ifosfamide 1800 mg/m2 infused over 1 hour, daily for 5 consecutive days, with mesna uroprotection, plus etoposide at 100 mg/m2 infused over 2 h on the first day). Courses were repeated every 3 weeks for a total of 4 cycles. He was further treated with EBRT to head and neck region to a dose of 50.4Gy which he tolerated well with significant reduction in facial and neck swelling (Figure 3). After NACT and preoperative RT, a repeat CT scan of face and neck was done for local response assessment and a FDG WB-PET scan was done to rule out any distant progression of disease. Though FDG-PET suggested a localized disease with partial metabolic response however CT suggested a good response and an operable disease as per the oncosurgical assessment. He underwent left maxillectomy and left MRND but per-operatively surgeons could not achieve an R0 resection as gross total excision of the intracranial component was not possible. The disease extent in our patient was not amenable to brachytherapy though this is a known modality which has been used in patients with good response to NACT. He developed biopsy-proven loco-regional recurrence within 14 weeks post-surgery with swelling of left side face and proptosis (Figure 4). He was put on adjuvant VAC/IE regimen, but after two cycles developed unresectable multiple pulmonary metastasis and systemic treatment was changed to VAC regimen. Subsequently within a week he presented with complaints of severe headache and vomiting. CT scan head revealed multiple brain metastasis for which he was given palliative whole brain RT to a dose of 30Gy. However, within 12 days he reported with severe low back ache and CT scan spine showed vertebral metastasis (Figure 5). He was treated with palliative EBRT to a dose of 30Gy in 10 fractions. In view of progressive disease, he was started on second line systemic chemotherapy cisplatin and etoposide. However, his condition kept on deteriorating and he finally succumbed to his illness within a span of 17 months after being diagnosed. We further discuss various aspects of adult RMS in a primary and metastatic setting based on the above case encountered by us.
Non-orbital RMS of head and neck region is grouped into parameningeal sites which include nasal cavity, nasopharynx, paranasal sinus (PNS), middle ear, pterygopalatine fossa and infratemporal fossa and non-parameningeal locations. These tumors have a propensity for invasion of base of skull and central nervous system (CNS) and have poorer survival outcome than orbital or non-parameningeal tumors. RMS is divided into the embryonal subtype which constitutes 60-80% being the most common. The botryoide type carries a better prognosis while the alveolar forms are more common among adolescents, arise in the extremities, and carry a worse prognosis [9]. Pleomorphic tumors arise mostly in deeper tissues are rare in children [10]. The pleomorphic subtype and RMS NOS are mostly seen in adult patients [4]. Truncal and extremity tumors are more common in adults with the relative proportion of alveolar tumors increasing with age, as a result, older age has often been perceived as an adverse predictor of survival. Nodal involvement appears to be an adverse prognostic factor in both pediatric and adult RMS [10]. Age has been identified as an independent predictor of prognosis, with children <1 year and >10 years having inferior survival [8]. In most studies, older age has been associated with morbid outcome and available literature suggests that prognostic variables like alveolar histology, regional and distant dissemination are more frequent in adults than in children [11]. Another independent poor prognostic factor described is the number of metastatic sites as was seen in our case with three sites of dissemination in a single patient within a span of 17 months [12]. Thus, multiple adverse clinical factors seem to converge in adults with RMS, and yet little is known about the disease. Predominance of unfavourable histological subtype like alveolar RMS in adults has shown significantly poorer metastases-free rates than embryonal and pleomorphic sub-types but this difference has not translated into significant disease-free survival [13].
Patients with RMS generally present with an asymptomatic mass or with signs and symptoms of mass effect or obstruction associated with primary tumor’s location. Parameningeal tumors may present with nasal obstruction, cranial nerve palsies, headache or facial swelling. Pathological evaluation has come a long way over years starting from a mere H&E stain deploying morphological examinations to incorporating IHC and further molecular testing [14]. IHC plays an important role in differentiating between a primary RMS and other pathologies with small round cell tumor appearing at similar head and neck site like basaloid squamous cell carcinoma, salivary gland tumors with high grade transformation, poorly differentiated synovial sarcoma, Ewing’s sarcoma, lymphoma, melanoma, olfactory neuroblastomas, or even metastasis from lung. On IHC analysis, the tumor cells react positively with desmin, actin and myogenin. Markers like TTF-1, CD-99, LCA, S-100 and melan-A were negative thus ruling out other pathologies and metastasis thus confirming the diagnosis of alveolar RMS of PNS. Alveolar RMS generally shows specific translocation t(2;13)(q35;q14) or t(1;13)(q36;q14) indicating rearrangement of the FKHR gene into either PAX3-FKHR or PAX7-FKHR gene [5]. However, abnormalities of FKHR with or without these translocations have also been identified in adults [15].
Adult RMS has poor prognosis with high propensity for local and distant dissemination to lymph-nodes, lung, skeletal system and brain [4-6]. However, sporadic cases of unusual dissemination to testes, breast, subcutaneous tissue, and pancreas are also present [16]. Paediatric alveolar RMS have been described to have a predilection for pancreatic metastasis [17] which was also seen way back in 1969 in an autopsy series by Enzinger, et al., who reported 67% pancreatic metastasis. Metastatic disease, whether upfront or dissemination during definitive treatment is associated with dismal prognosis both in children and adult populations [18]. The presence of metastatic disease at presentation has been significantly associated with mortality on both univariate and multivariate analysis [10]. Correlation between 5-year survival and metastasis at presentation has been reported by Esnaola NF, et al., to be 31% and 25% respectively; La Quaglia M, et al., 56% and 23%; the IRS-I 56% and 17%; IRS-II 65% and 19%; and Lloyd R, et al., 21% with upfront metastatic disease[10,19-22]. Further, dissemination of disease while on systemic therapy or RT has been associated with poor final end-point in several paediatric and adult RMS series [19,22,23]. In patients with distant metastatic disease, chemotherapy dosages were stepped-up in the IRS-I, IRS-II and IRS-III studies resulting in early remission and improved survival. Based on these improved outcomes from IRS studies, high-dose systemic therapy along with hematopoietic-cell rescue has been in used in recurrent and metastatic paediatric RMS [24]. Worse survival has been documented with univariate and multivariate analyses showing poor survival in patients with tumor size of more than 5 cm [19], as was seen in our case also. Increased tumor size has been associated with poor response to systemic therapy and subsequent distant metastasis [9]. Alveolar RMS seen mostly in young adults as compared to paediatric age-group has the highest incidence of lymph-node involvement. Regional lymph nodal involvement has also been found to be a significant predictor of survival and distant metastasis with rate of nodal disease being 28% [19]. However, few studies did not find any correlation between nodal status and metastatic state or survival treated with multimodality therapy [9]. RT has been used as a palliative treatment option for bone metastasis with significant improvement of painful symptoms. Solitary lung metastasis may be surgically resected in case of an oligometastatic scenario with the primary disease under control.
Adult RMS is an uncommon disease and there is scarcity of relevant guidelines regarding the optimal management protocol and prognosis of these patients due to absence of prospective clinical data. The current guidelines for treating adult RMS are based on IRS which achieved striking improvements in long-term survival for children with RMS [20,21,25,26]. Initial induction chemotherapy has been traditionally used for pharmacologic debulking to allow for a more conservative surgical approach or less aggressive RT. Induction chemotherapy used upfront for head and neck RMS have always been followed by RT, however with or without salvage surgery because of poor response or recurrence. Chemotherapy has shown similar activity in paediatric and adult RMS, the final outcomes between both groups are comparable when similar chemotherapy regimens are administered [10,13,27]. Adults RMS have demonstrated an overall response rate of 85% to chemotherapy was 85% when treated on same lines as that of paediatric RMS, however Hawkins, et al., in their study in 2001 postulated no survival benefit in adult patients treated with chemotherapy used in paediatric cases [27,28]. Interaction between radiation and some of the commonly used chemotherapeutic drugs like dactinomycin and doxorubicin can produce undesirable adverse effects and is therefore usually avoided. However, vincristine and cyclophosphamide can be given concurrently with RT [8]. Loco-regional recurrence is the most common pattern of failure and therefore surgery and RT play important roles in the treatment of these cases. Radical resection may cause undue morbidity in PNS locations and therefore brachytherapy may be employed with success in selected patients [8].
Systemic therapy for RMS may be different than those used for other STSs. Vincristine, actinomycin-D or dactinomycin and cyclophosphamide (VAC) has been the standard chemotherapy regimen for paediatric non-metastatic intermediate and high-risk RMS [29]. The addition of topotecan to VAC regimen did not improve FFS in intermediate-risk patients as was concluded in IRS-V study [8,29]. In adults vincristin and dactinomycin has been used with or without cyclophosphamide as those treated with the two drug regimen and three drug regimen had similar FFS rates [29,30]. Other regimen used in adults have been vincristine, doxorubicin and cyclophosphamide either alone or alternating with etoposide and ifosfamide in intermediate-risk patients [31]. We used VAC alternating with IE in view of high-risk disease and later changed to VAC as he metastasized. Various chemotherapy regimens mentioned above have been used in various retrospective series for adult RMS, however no improvement in OS was achieved. Second line systemic therapy with cisplatin, carboplatin and etoposide has been exhibited in adults without any appreciable improvement in OS, DFS or metastasis free survival (MFS) [13,27]. We used cisplatin and etoposide after the patient progressed on VAC/IE and then on VAC, however the regimen failed to provide any therapeutic advantage. Few non-randomized trials have used high-dose chemotherapy with stem-cell transplant in paediatric RMS only with miniscule success however other studies have failed to show any benefit with this protocol [32,33].
Advent of novel targeted therapies and pharmaceuticals in addition to chemotherapeutic agents has added a new dimension to the management aspect of RMS particularly in children but unfortunately such measures have failed to provide any survival advantage in adults. Molecularly targeted agents against VEGFR receptors expressed in RMS, against erbB-2, EGFR and mTOR pathway in both embryonal and alveolar paediatric RMS has been used in few trials [34-36]. PDGFR-A has been reported to be a mediator of disease progression and metastasis in alveolar RMS. Inhibition of PDGFR-A by imatinib, both in vitro and in vivo has established this principal as a potential therapeutic target in alveolar RMS [37]. However, since the survival rate for metastatic alveolar RMS has remained unchanged for decades, it is unlikely that a single targeted therapy will be sufficient for long-term survival. Hh signalling is one of the molecular mechanisms often deregulated in carcinogenesis and specifically involved in the tumorigenesis of RMS and has been associated with poor prognosis, however targeted agents directed against this pathway may be a promising therapeutic strategy for RMS in future [38,39]. The IGF-1R-PI3K pathway is widely over-expressed in RMS and has been described to be the reason for resistance to Hh inhibitors, although rapamycin, an mTOR inhibitor, has been reported to target RMS tumor cells by inhibiting both PI3K/AKT/mTOR and Hh pathways [40,41]. The inhibitory effect of GANT61 on a component of the Hh signalling in RMS cell lines known as glioma-associated oncogene (GLI), is enhanced by combining mTOR inhibitors and systemic chemotherapeutic agents, thus suggesting that management protocols using Hh inhibitors might be a beneficial therapeutic modality [42]. Since RMS cells are unable to complete skeletal muscle differentiation, restoration of this differentiation program would be a novel anti-RMS chemotherapeutic approach for the treatment of metastatic RMS, especially alveolar subtype. Increased levels of epigenetic modifier histone H3 lysine-9 methyltransferase Suv39H-protein (KMT1A), an antimuscle differentiation mechanism in alveolar RMS cells has been identified as a prospective target for developing pharmaceuticals for restoration of differentiation in paediatric alveolar RMS cases [43]. Alveolar RMS cases generally express N-myc which augers poor survival in these patients and therefore can act as a target for antagonistic therapy [44]. Celecoxib has been explored in targeting STAT3, an important signaling pathway in the oncogenesis of RMS while preclinical study with Drozitumab against death receptor DR-5 expressed on many RMS cells has shown early promising results [45,46].
Aggressive surgery is rarely indicated in these parameningeal sites because approximately 20% of these tumors are located at sites where complete resection is usually not possible [7]. Surgery also does not obviate the need for RT, however due to post-operative complications may delay systemic chemotherapy [5]. Surgical approaches to these head and neck RMS with intra-cranial extension involves multi-speciality skull-base surgery. With the main goal of preservation of function and cosmesis, reasonable surgery involves removal of bulk of tumor with maximal conservation of anatomic structures [8]. Radiation is necessary to ensure local tumor control in patients who are unable to undergo complete surgical resection. Local control of gross disease in most cases require dose of 50 to 55 Gy. Omission of RT post-induction chemotherapy or surgery may result in inferior survival [7]. The dosage of EBRT depends on the stage and site of lesion. The recommended dose for microscopic residual disease is 36 Gy - 41.4 Gy and for gross residual disease it is 50.4 Gy [26]. We used dose of 50.4 Gy at 1.8 Gy per fraction in view of stable disease post induction chemotherapy. RT improves local control but not OS due to the heterogenous nature of adult RMS. Generally to reduce post-RT side effects, cone-down can be done based on tumor response to induction chemotherapy with significant reductions in high-dose RT treatment volume while maintaining excellent tumor control [47]. In patients with parameningeal location and intracranial extension, initiation of early RT with wider planning margins to cover adjacent sites which are at higher risk of meningeal extension is very important for adequate disease control [48]. High-dose methotrexate has been used in selected paediatric patients with CNS involvement who are unfit for RT [49]. However, such therapy in adults is not known. Use of RT with concurrent carboplatin and irinotecan has been used in children with intermediate to high-risk cases [50]. The timing of RT is coordinated with chemotherapy to optimize local control and drug doses. RT is generally delayed for 6-12 weeks to allow administration of neoadjuvant chemotherapy. However, earlier irradiation, especially in high risk patients may provide better local tumor control and survival [51]. For intermediate-risk patients there are studies to test the effect of delivering RT early in week 4 concurrently with irinotecan [8]. Convetional RT was replaced with hyperfractionated accelerated RT in CWS-86 study. This study used RT dosage of 1.6 Gy twice daily to a total dose of 32 Gy in a good responders and 54.4 Gy in a poor-responders to induction chemotherapy with satisfactory local control. The CWS-91 study further reduced the RT dose in poor-responders to 48 Gy with improved local control and EFS [8]. But unfortunately, all these RT schedules have been applied in paediatric patients with no similar exhibition in adult patients which has added to the dismal outcomes in affected adults.
Paediatric patients with localized disease are currently being cured using multimodality treatment [52]. Unfortunately, adults with RMS continue to have a very dismal prognosis with OS rates of only 20%-40% [27]. This poor OS may suggest that factors other than an unfavourable clinical presentation are involved in the unsatisfactory outcome of adult RMS patients. Although major differences in cellular biology and pathophysiology between paediatric and adult RMS have not been described till date, increased expression of multidrug-resistance proteins in adult RMS as compared to children may be a possible culprit apart from the lower tolerance of adults to intensive chemotherapy regimens [53]. Information regarding clinical and biologic characteristics of adult RMS is very limited due to its rarity. Large multi-institutional randomized controlled trials have not been performed, and only literatures from single institutional retrospective studies are present. Despite the recent use of multimodal therapy used in these studies, the prognosis in older patients with alveolar RMS appears to be worse. Survival is significantly better in patients who respond to chemotherapy than those who do not, although early sensitivity to chemotherapy in patients with metastatic RMS does not necessarily translate into cure [12]. Further studies on treatment variables and biology of adult RMS are clearly needed to further clarify differences between adult and paediatric RMS. The biological behaviour and prognosis of adult RMS is still poorly understood due to the limitations of various retrospective studies and lesser number of patients treated. Distant metastasis remains a harbinger of dismal prognosis with upfront metastatic state and poor response to chemotherapy being independent predictors. Localized alveolar RMS of PNS should therefore be treated aggressively with multi-modality approach comprising of chemotherapy, RT and surgery with primary goal of cure and improve quality of life of patients, emphasising on preservation of function and cosmesis. We emphasize that as conventional chemotherapeutic and radiation strategies have repeatedly failed to succeed in metastatic alveolar RMS in adults as was experienced by us, there should be an urgent need of development of altered fractionation schemes, novel targeted therapies and pharmaceuticals to treat this dreadful disease.
We extend our gratitude to SAJ Cancer Science publishing group for providing complete waiver of processing and publication fees.
We thank the patient and his relatives for allowing us to publish his case and use the images taken during his stay in hospital.
We also like to thank department of Surgical Oncology, Pathology, Radiology and Nuclear Medicine, Army Hospital (Research and Referral), New Delhi, and Command Hospital (Southern Command), Pune, India.
The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.