Antibiotic Resistance Involves Antimicrobial Inactivation in Global Communities
The science of ageing has become of critical interest to the antimicrobial drug industry with aging and geriatric medicine associated with antibiotic resistance and mitochondrial apoptosis with relevance to the global chronic disease epidemic. In the United States and Europe the geriatric population (> 65 years) is expected to double by the year 2060 with the death rate in the European Union in the geriatric population to be greater than 80% when compared with individuals <65 years [1]. Antimicrobial resistance has led to increased medical costs with the emergence of new antibiotic resistant infections in the United States and other countries [2-10]. The worldwide overuse of antimicrobial drugs and lack of new antibiotic agents has led to a major problem with healthcare in various diabetic and geriatric communities. Antibiotic resistance has led to assessment of preventive strategies associated with newer drug development to assist clinicians in the treatment of diabetics, geriatrics and Alzheimer’s disease individuals [2-10].
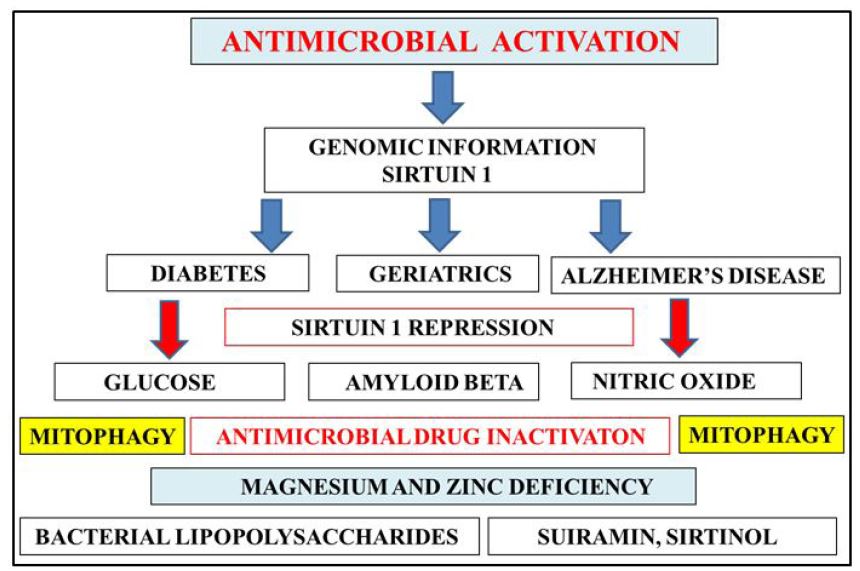
Genomic information has become important to antibiotic resistance and genomic medicine is now important as a preventative strategy to increase antibiotic treatment in aging and geriatric individuals (Figure 1). In diabetic individuals the inability to control plasma glucose levels has been associated with inappropriate antibiotic use relevant to poor glycemic control and the treatment of antibiotic-resistant infections [5]. In aging and Alzheimer’s disease the amyloid beta peptide has become of critical interest to antimicrobial drugs with its therapeutic role as a natural antibiotic [11,12]. The connections between glucose dyshomeostasis and amyloid beta aggregation has been documented in cells from obese and Alzheimer’s disease individuals [13-15]. Novel genomic information now indicates that the gene Sirtuin 1 (Sirt 1) that regulates the suprachiasmatic nucleus is important to plasma glucose and amyloid beta metabolism with Sirt 1 inactivation connected to the inappropriate hepatic amyloid beta, glucose, drug metabolism associated with drug-drug interactions and inactivation of antimicrobial therapy (Figure 1) [16-22].
The diabetes severity index is now related to the inactivation of Sirt 1 with Sirt 1 relevant to senescence in diabetes, geriatrics and Alzheimer’s disease [23]. Sirt 1 and its antimicrobial properties involve nitric oxide (NO) with Sirt 1 inactivation involved with NO dyshomeostasis and mitophagy. The use of antibiotics in aging and diabetes may require NO homeostasis and an intact immune system with Sirt 1 inactivation in aging, diabetes and AD associated with antibiotic resistance relevant to toxic immune reactions and mitophagy connected to global chronic disease (Figure 1) [20,24]. The gene Sirt 1 and its repression is regulated by the bacterial lipopolysaccharide (LPS) that are released from Gram negative bacteria [25]. LPS is critical to glucose dyshomeostasis and amyloid beta aggregation with elevated plasma LPS levels associated with non alcoholic fatty liver disease relevant to inactivation of antimicrobial drug therapy [18-21,25,26]. Antimicrobial drug therapy is associated with increased bacterial LPS and amyloid peptide release from gram negative bacteria with Sirt 1 inactivation associated with bacterial amyloid peptide induced amyloid beta aggregation [27,28].
Nutritional therapy is essential for antimicrobial activation and the therapeutic use of antibiotics. Diet that regulate NO are essential to prevent antimicrobial drug inactivation [29]. Low fat diets that do not allow LPS absorption are essential to prevent inactivation of antimicrobial use [30]. Sirt 1 inhibitors such as suramin and sirtinol should be carefully regulated to prevent complete Sirt 1 inactivation [31]. Caffeine as an antimicrobial/Sirt 1 modulator and various Sirt 1 activators should be consumed to prevent antibiotic resistance. LPS and its effects on magnesium and zinc deficiency should be carefully assessed to prevent antimicrobial drug inactivation and mitophagy [20,31-34].
This work was supported by grants from Edith Cowan University, the McCusker Alzheimer’s Research Foundation and the National Health and Medical Research Council.
The major interest in genomic medicine in healthcare in various communities with relevance to antibiotic resistance has led to early plasma Sirt 1 analysis as an assessment for preventive strategies associated with continued therapeutic antibiotic use with relevance to genomic medicine development in aging, diabetes and Alzheimer’s disease. Nutritional therapy is essential to maintain antimicrobial activation with relevance to mitophagy and the global chronic disease epidemic.