Antimalarial Phytochemicals: Delineation of the Triterpene Asiatic Acid Malarial Anti-Disease and Pathophysiological Remedial Activities - Part I
Malaria is a composite condition of the Plasmodium parasite infection and accompanying pathologies. Parasite induced red blood cell perturbations and immunological response to infection drive various organ-specific syndromes accounting for a huge percentage of deaths amongst children <5 years and pregnant women. The multi-factorial pathophysiology includes acute renal failure, hypoglycaemia, severe malaria anaemia, acute respiratory distress syndrome/ acute lung injury and cerebral malaria as some of the prominent presentations of the disease. Current malaria treatment has largely remained “anti-parasite” or “anti-infection” necessitating discovery of “anti-disease” drugs that will ameliorate immunological aberrations, inflammation and metabolic disturbances which are ultimately the cause of high morbidity and mortality. Asiatic acid, a phytochemical, has well known curative properties on other conditions which share disease manifestations with malaria. However, the influence of Asiatic acid on malaria has not yet been reported. This review unravels the different facets of Asiatic acid and their possible remedial effects on molecular and biological changes introduced by the disease with emphasis on how this relates to glucose metabolism, acute renal failure, severe malaria anaemia, acute respiratory distress syndrome/ acute lung injury and cerebral malaria.
Keywords: Asiatic Acid; Phytochemical; Malaria; Anti-Disease; Antioxidant; Ant-Parasitic; Anti-Disease
List of Abbreviations: AA: Asiatic acid; ARF: Acute Renal Failure; SMA: Severe Malaria Anaemia; ARDS/ALI: Acute Respiratory Distress Syndrome/Acute Lung Injury; CM: Cerebral Malaria; Nra: Non Respiratory Acidosis; NO: Nitric Oxide; iNOS: Inducible Nitric Oxide Synthase, ONOO-: Peroxynitrite; ROS: Reactive Oxygen Species; OS: Oxidative Stress
Malaria is a complex haematological disease initiated and driven by parasitological agent, the Plasmodium parasite, with accompanying multifaceted pathophysiology. Red blood cell (RBC’s) morphological and functional changes observed in malaria and immunological response to infection orchestrate organ-specific syndromes accounting for high morbidity and mortality among children <5 years and pregnant women in malaria-endemic areas [1-4]. The clinical sequelae include acute kidney injury (AKI), hypoglycaemia, severe malaria anaemia (SMA), acute respiratory distress syndrome/ acute lung injury (ARDS/ALI) and cerebral malaria (CM) as some of the prominent presentations of the disease [5-14]. Current malaria treatment is “anti-parasite” or “anti-infection”. This makes the discovery of new “anti-disease” drugs that will ameliorate the pathophysiological manifestations which are ultimately the cause of high incapacitations and death rates. Asiatic acid (AA), a phytochemical, has well known curative properties on other conditions which share disease manifestations with malaria [15-18]. Reports on the influence of AA on malaria are slowly emerging. Administration of the phytochemical has been mainly oral or as topical ointment for wound healing. Systemic circulation administration through transdermal delivery systems (TDDS) has been added to its list of administration options. We would like to explore the potential therapeutic effects of AA on molecular and biological changes introduced by the malaria disease emphasizing on how this may relate to glucose homeostasis, AKI, SMA, ARDS/ALI and CM with infusion of methods of drug delivery to flavour the mix.
Triterpenes are synthesized through the combination of 6 isoprene units (C5H8) [19]. These are a group of phytochemicals which characteristically exhibit pentacyclic compounds with substitutable functional groups enabling them possible interaction with a variety of substances. Natural triterpenes are secondary metabolites of plant species with selective oxidant and antioxidant properties [20]. Triterpenes have been suggested to possess oxidative properties, in the same way with artemisinin and analogous antimalarial pharmaceuticals [21]. This way, triterpenes may mimick the evolutionary effects of haemoglobinopathies (glucose-6-phosphate dehydrogenase deficiency, sickle cell disease) on the infected red blood cell (pRBC’s) environment that kill the parasite by altering its oxidative status [22,23].
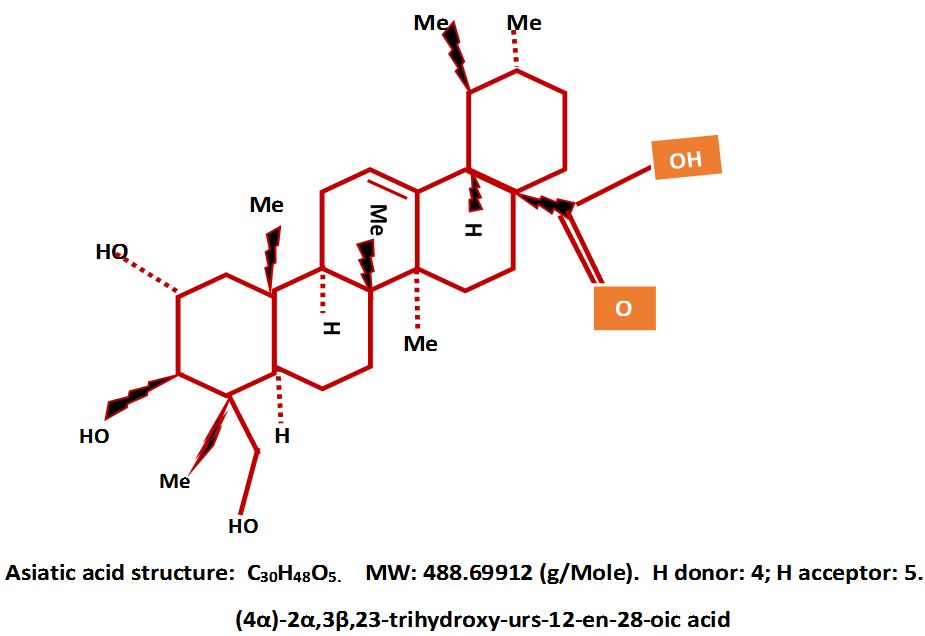
Asiatic acid is a pentacyclic steroidal triterpene derived from the perennial herbaceous creeping plant Centella aciatica (Apiaceae). The phytochemical is also found in the glossy privet fruit, Ligustrum lucidum, in basil, Ocimum basilicum, and in brown mustard, Brassica juncea [25].
The hydrophobic nature of AA and its accompanying functional groups of one carboxyl, three hydroxyls, six methyl groups and three hydrogen’s makes the compound to have both antioxidant and oxidative properties. The molecular weight (MW) of AA qualifies it for drug classification in the chemotaxonomic group of triterpenoids (Figure 1) [26,27].
The molecule is amphiphilic with a hydrogen bond donor (HBD) of 7.1 and a hydrogen bond acceptor (HBA) of 4.172 indicating its capacity for redox reactions with its antioxidant potential more than its oxidant capacity [28,29-31]. The pharmacophore of AA lies in the A ring and the hydroxyl groups found at position C2α, C3β, and C23. The tri-hydroxyls and the carboxylic group at C28 are suitable for anti-oxidative and anti-inflammatory activities suitable for the amelioration inflammatory conditions [32,33].
A -11.8122 kcal/mol docking (binding) energy of AA onto 3-hydroxy-3-methyl glutaryl-coenzyme A reductase (HMG-CoA), the rate-limiting enzyme in the synthesis of cholesterol, has the capacity to inhibit its enzymatic activity [34]. This implies that AA may have an influence on cholesterol metabolism. The PreADMET program has been used to predict the absorption, distribution, metabolism, excretion (ADME) and the Toxtree software has been used for toxicity (T) prediction of AA by some researchers [35]. AA percentage human intestinal absorption (% HIA) was predicted as 91.23%, Caco-2 cell permeability as 20.97nm.sec-1 and plasma protein distribution as 96.45%, interpreted to mean well absorptivity, mild permeability and strong binding, respectively [31].
Binding of AA to human serum albumin (HSA) is critical as it determines the pharmacokinetics of the phytochemical and its bioavailability, distribution, metabolism, excretion and therapeutics or toxicity index [36-38]. Computational auto-docking simulation and fluorescence quenching experimental studies of HSA-AA interaction has been shown to occur through mostly hydrophobic and a few hydrophilic bonding giving the structure high integrity and rigidity with a free energy (ΔG) of -6.36kcalM-1, and -6.23kcalM-1, respectively [39]. This means changes in HSA levels may influence pharmacokinetic and therapeutic efficacy of AA and making drug bioavailability linked to the HSA half-life, a factor worth noting in SMA.
Other researchers have reported, however that low levels of plasma AA seen in beagle dogs after an oral dose of a C. asiatica extract could be a result of poor intestinal absorption, where T1/2 = 4.29h, Tmax= 2.7h, Cmax = 0.74μg/mL were observed [40]. Most AA is metabolized to glucuronide or sulphate metabolite with a small percentage of the free drug found in plasma [41].
Indigenous medicinal practice rates the plant Centella asiatica’s product, AA, as a learning and memory booster intimating possible cerebral malaria protection. In Asian and African countries the plant is used for treatment of wounds, ulcers, arthritis, depression and anxiety [42-45]. Other major triterpenoid sugar esters, with their corresponding aglycon counterparts, are considerable pharmacologically active principals with well-established medicinal effects in vitro and in vivo [15].
In vitro and in vivo AA properties include antioxidant, anti-tumourogenesis, hepatoprotective, anti-inflammatory, neuroprotective, antihyperlidaemic, anti-hyperglycaemic, haemodynamic and metabolic modifiers [34,46-54]. Protection against neurodegenerative glutamate-induced brain injury and cytotoxicity towards many types of cancer cells has been explored [56,57]. AA was observed to induce apoptosis and cell arrest in human breast cancer through the activation of extracellular signalling kinase (ERK) and p38 mitogen-activated protein kinase [p38MAPK] [58]. Anti-glycative effects of AA on human keratinocyte cells acting through anti-oxidant, -apoptosis, and -inflammatory activity by lowering ROS production, caspase activity downregulation, receptor for advanced glycative end-products (RAGE) suppression, activation of p38 and/or JNK and decreasing expression of matrix metalloproteinases (MMPs) was reported [59]. Similar processes do occur in malaria indicating that the phytopharmaceutical AA may play a major role in the treatment of the disease.
With the many functions of AA observed so far, its effect on malaria is just starting to be explored. In our laboratory, treatment of P. berghei murine malaria infected Sprague Dawley (SD) rats with AA (5mg, 10mg and 20mg/kg) by both oral and transdermal route administration and as prophylactic and therapeutic agent has been reported [60-63]. The influence of AA on glucose homeostasis preservation in murine malaria animal model has also been reported [64]. However, the mode of action of the drug on malarial treatment requires further exploration.
We premise the mode of action underlying parasitic eradication of AA on its known properties in diseases with a common pathophysiological pattern to malaria. The known pathophysiology of malaria needs to be examined to see how AA is involved in malaria disease resolution. Naturally, the postulation that AA, as a natural plant secondary metabolite, has possibly coevolved with the eternal malaria matrix-mix (humans, mosquitoes, malaria parasites) to arbitrate a mutual coexistence without drastic erosion of the each member’s sphere of influence within the ecological milieu, may be correct. The proposition is that, if the Plasmodium parasite did not cause disease in the human being, like the commensal microbial population in the gut, there will be no need to seek its eradication. If AA was able to render the body successfully able to resist malaria disease outbreak and progression through maintenance of suboptimal levels of the parasite in the body, a phenomenon occurring in the immune competent populations, malaria interventions may not be necessary [65,66].
Genetic mutations induced by the malarial disease on certain world populations protecting them against infection (G-6-PD deficiency and Sickle Cell Trait) seem to be the extreme responses by the human being to an inherent parasitic infection in the absence of a possible anti-disease co-evolutionary component [65]. The protection offered by these genetic variants has varied untoward outcomes and diseases more debilitating than malaria. Medical emergencies with far-reaching implications and fatalities have been observed in the homozygous individuals for sickle cell disease [67,68].
In many body organs the Plasmodium parasite does not cross blood vessel endothelium into the surrounding tissues for effective infectivity. The Plasmodium exerts its “contamination” of body systems through RBC invasion and destruction, vascular occlusion, host inflammatory system manipulation and metabolic derangements. These processes are mediated by components and products from RBC destruction, microvasculature homeostasis, and immune system responses. The abnormalities are common in other diseases which are not necessarily linked to malaria. AA has been shown to alleviate the same effects of malaria in other disease processes and the mechanism by which it confers corrective measures may also apply in malaria [69, 70].
Inflammatory processes in the pathogenesis of many parasitic and metabolic diseases are critical. The number of inflammation mediators identified, to date, include platelet-activating factor (PAF), cytokines, chemokines and adhesion molecules which facilitate targeted inflammation cascading [71]. There is ample evidence that phytopharmaceuticals may modulate various inflammatory mediators, control the production and action of second messengers, direct the expression of transcription factors and key pro-inflammatory components [72,73]. The underlying mechanism of anti-inflammatory action for AA in malarial may include: (a) anti-oxidative and radical scavenging; (b) inflammatory cellular components modulation (macrophages, lymphocytes neutrophils); (c) modulation of expression and/or activity of pro-inflammatory enzymes such as phospholipase A2 (PLA2), cyclooxygenase (COX), lipooxygenase (LOX), inducible nitric oxide synthase (NOS) and (d) modulation of pro-inflammatory gene expression [69]. AA Interaction with these biological systems require critical exploration.
In malaria inflammation, the immune system-triggering-malaria-toxin has been identified as glycosylphosphatidylinositol (GPI) which is released into the circulation when pRBC rupture shortly after erythrocytic schizogony [74]. GPI initiates TNF-α and lymphotoxin (formerly TNF-β) production [75], up-regulates ICAM-1 and IVCAM-1 [76,77]. Haemopoietic mediators of inflammation include Th1/M1 cytokines mainly TNF-α, IL-1, IL-6, IL-18 and Th2/M2 cytokines interleukin-4 (IL-4) and interleukin-10 (IL-10). When produced excessively as in severe malaria, Th1 cytokines may lead to the generation of fever, hypoglycaemia, bone marrow suppression, coagulopathies, hypergammaglobulinaemia, hypotension and elevated acute phase reactants [78,79]. The works by Clark and Chaudhri (1988), showing that TNF-α induced dyserythropoiesis and erythrophagocytosis in malaria-infected animals, proved the association of SMA to inflammatory mediators and corroborated Peetre, et al. (1986) who demonstrated growth inhibition of culture haemopoietic cells [80].
Taken together, the anti-inflammatory effect of AA may modulate pro-inflammatory components in malaria just like it does in diseases characterized by the same inflammatory processes. Indeed, AA facilitated selective mitochondria-dependent apoptotic activity on activated Th1 cells preventing concanavalin (Con-A) induced murine fulminant hepatitis in a dose-dependent (10 and 20μg/kg AA) fashion that disrupted mitochondrial transmembrane potential, released cytochrome c, activated caspases and cleaved poly(ADP-ribose) polymerase [PARP] [81]. The haematological differential counts usually display exaggerated lymphocytosis in malaria. As inflammatory response is similar regardless of cause, AA may modulate Th1 over expression in malaria by eliminating activated cells. Moreover, in a mouse model for pain and inflammation, AA was able to block the activation of NF-kβ [82], a major transcription factor in the regulation of pro-inflammatory cells, cytokines and enzymes [83].
In unstimulated Th1 cells, NF-kβ subunit p65/p50, is sequestered in the cytosol bound to the inhibitory factor Ikβ-α. Pro-inflammatory signals in malaria including GPI, cause the phosphorylation of Ikβ-α by Ikβ kinase (IKK) and its inactivation though the ubiquitin-mediated degradation. Freed, NF-kβ translocate into the nucleus acting as pro-inflammatory mediator and transcription factor [84-86]. Termination of the inflammatory response is critical for overall health maintenance and triterpene AA may be able to inhibit GPI production or maintain inactivation of NF-kβ or both as this anti-inflammatory mechanism has been shown in other diseases other than malaria when treated with madecassoside (MA), a similar triterpenoid to A-A [87-89].
The ability of AA to inhibit activation of NF-kβ may suggest also subsequent iNOS and COX-2 inhibition and reduction in nitric oxide (NO) release [87]. Indeed, AA (10mg/kg) injected into Carrageenan-induced paw oedema inhibited expression of iNOS, COX-2 and NF-kβ in mice [82]. In malaria this may mean reduction in uncontrolled vasodilation associated with vascular permeability, pulmonary oedema or renal dysfunction. Toxic oxidative reactions which cause tissue injury may also be ablated by a reduction of NO and possibly superoxide [O2•-] [90]. Indeed, AA has been predicted by a computational model AutoDock v.3.05 to bind iNOS, inhibiting binding to arginine, with strong affinity displaying free energy binding (FEB) of -9.79kcal.mol-1 [30,31,35]. Chemoattractant mediators which depend on NF-kβ activation may also be inhibited resulting in abrogation of neutrophil-aggregation and inactivation of the associated oxidant and pro-inflammatory injury lytic enzymes [91]. Activation of peroxisome proliferator-activated gamma (PPAR-γ) which regulates inflammation through NF-kβ translocation inhibition may be a pathway by which AA induces an anti-inflammatory activity. This process has been demonstrated with curcumin, a multi-faceted phytopharmaceutical [92]. The subsequent action of this activation will be up-regulation of CD36 in monocytes/macrophages for non-opsonic pRBC’s phagocytosis with possible parasite extrusion or destruction [93].
Demonstration of AA anti-inflammatory capacity in malarial pathophysiology may lend credence to the postulation extended here that phytochemicals evolved naturally to counter-balance malaria pathogenesis seeing that these plant constituents are natural food stuffs. Indeed, the anti-inflammatory effect of AA has been reported in a murine malaria model where C - reactive protein was shown to be significantly reduced in infected transdermal AA administered animals as compared to infected chloroquine-treated animals and non-treated controls [94]. Therefore, studies that may show how AA anti-inflammatory processes are affected in malaria are required.
As in other pathologies that cause increased oxidative stress, malaria disease orchestrates multi-organ injury and degeneration through the reactive oxygen species (ROS) destructive effects. Oxidative stress (OS) plays a pivotal role in pRBC’s contribution to disease manifestation including pRBC’s sequestration, AKI, CM, SMA and ARDS [95,96]. The defence mechanism of ROS and NO against disease and their signal transduction capacities makes them have a both beneficial and pathological role which necessitates regulation in malaria. However, these facets need to be completely elucidated in further malarial research.
A number of possible sources of ROS exist in malaria where AA may play a role in reducing. Plasmodium parasites have diminished capacity to mobilise amino acids and depend on haemoglobin (Hb) breakdown. Haeme, a highly toxic Hb breakdown compound, increases OS even at very low concentrations. Ferri/ferroprotophorphyrin IX (FP) in Hb-free haeme generates OS environment. Haeme requires detoxification to hemozoin (β-haematin), its biocrystalization or biomineralization product [97,98] or it will oxidise parasite food vacuole membranes. Chloroquine and other 4-aminoquinolines use this mechanism to disrupt parasite survival by inhibiting haeme biocrystalization in pRBC’s and increasing OS [99-102].
The host cell immune response to parasitaemia also yields to the pRBC a high overall oxidative burden. However, the parasite has evolved mechanisms for increased antioxidant capacity, which may only be overcome by a highly oxidative agent like AA [103-105]. Generation and targeting of ROS on inhibiting specific parasitic enzymes or membranes, as occurs with endoperoxidase antimalarials (which share similar characteristics with AA), have higher chances of clearing parasitaemia [106]. The site of AA activity on the malarial parasite is not yet known. Noteworthy, the food vacuole where an antioxidant environment is necessary for the parasite potency, the antioxidant capacity of AA may aid parasite propagation. Indeed, it is highly unlikely that the design of nature would have it to be so and the oxidative capacity remains the most probable cause of AA lethality to the parasite. The most probable scenario is that AA exerts an oxidative effect in the acidic food vacuole and an antioxidant effect on cellular OS, occasioned by the host immune response, rendering both anti-parasitic and anti-disease ameliorative facets.
Both enzymatic and non-enzymatic antioxidants have been shown to be up-regulated by an oral administration AA (20mg/kg) in streptozotocin-induced diabetic rats with subsequent prevention of lipid peroxidation [33]. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) together with ascorbic acid and reduced glutathione (GSH) were found to be elevated in the AA treated DM animals as compared to the untreated DM control [33,34,54]. Indeed, lipid peroxidation is common in severe malaria, therefore, antioxidant capacity of AA seen in DM may be inferred in malaria [107].
While the generally and previously accepted mechanism of tissue hypoxia in malaria has been attributed to vascular occlusion by agglutinated RBC’s, it has since been observed that the other mechanisms are also at play [108,109]. Sepsis, a systemic pathology like malaria, does not experience RBC’s sequestration yet there is a similar disease presentation owing to the same underlying mechanism of hypoxia. Tissue oxygen tension is usually normal or elevated in sepsis of the rat, patients or pigs [110-112]. Poor oxygen utilization and not supply is seen as the major cause of hypoxic conditions of malaria.
Mitochondria dysfunction results from excessive ROS, NO and especially ONOO-. Inflammatory cytokines-induced excessive iNOS expression by monocytes and macrophages increase NO and oxidative stress which reversibly inhibits cytochrome oxidase and aconitase [113-115]. Energy generation is subsequently reduced leading to respiration fatigue and hypoxia. However, it is worth noting that this process of energy depletion is not exclusive as poor oxygen delivery is also a critical way by which hypoxia may develop and also both routes may ensue simultaneously.
Poly (ADP-ribose) polymerase (PARP) is an enzyme that catalyses transfer of ADP-ribose units from β- nicotinamide adenine dinucleotide (NAD+) to a number of different proteins to produce linear and/or branched polymers. The nuclear enzyme, PARP, is activated by DNA strands breaks or nicks as a repair mechanism. Strands breaks may be initiated by oxygen free radicals or their NO reaction products ONOO- resulting in the depletion of NAD+ and conversely ATP, in an effort to restore the critical NAD+ [116-118]. This action invariably compromises aerobic respiration with the possibility of causing bioenergetics failure. Increased generation of ONOO-, as seen in inflammation through induction of iNOS and subsequently NO, activates over expression of PARP. Concomitantly, ATP rundown and mitochondrial dysfunction occur predisposing to polymyopathy, hypoglycaemia, hyperlactaemia which have been mostly attributable to poor oxygen delivery, and are common in malaria [86].
Prevention of PARP-induced energy rundown may comprise of increasing supply of NAD+, PARP activation-inhibition or quenching the inflammatory drivers of PARP activation, i.e. NO and ONOO- [119]. Supplementation with NAD+ in sepsis or malaria is somewhat unpractical whilst inhibition of PARP may have deleterious effects. In malaria, the anti-inflammatory and antioxidant properties of AA may play a role in the inhibition of the inflammasome and consequentially, PARP activation by ONOO-, leading to malarial disease amelioration. Moreover, inhibition of PARP activation by certain agents has been reported to protect against brain ischaemia, splanchnic ischaemia and reperfusion [120-123], lipopolysaccharide toxicity, local inflammation and brain pathology in mice, multi-organ failure in rats and sepsis in pigs [124-126].
The other intriguing factor in PARP is that transcription factor NF-kβ is associated with the enzyme’s activation. Involvement of NF-kβ in DNA repair, immune response and apoptosis places it centrally to the expression of genes essential to systemic inflammatory disease mainly, TNF-α, IL-1β, IL-6, IAM-1, E-selectin and iNOS [84,86,127-130]. Essentially, PARP activation through ONOO- as a result of inflammation, consumes NAD+ causing poor energy utilization and energy depletion [131]. Subsequently, hypoxia causes inflammation-induced tissue damage, increased inflammatory cytokines production and more ONOO- production. Ultimately, a vicious cycle of severe inflammation breeding more of itself leads to multi-organ failure, coma and death [86]. Thus anti-inflammatory mediators like AA may have essential roles, through inhibition of NF-kβ, in the alleviation of malarial disease which essentially is underscored by inflammatory processes. Other specific molecular targets for AA outside the inflammatory mediators are not well known. It is possible that the phytochemical may have a number of docking capabilities in noteworthy enzymes involved in malarial disease.
Erythropoiesis suppression occurs during the pre-patent period (72 hours post infection) in murine malaria. This is a possible host protective mechanism against the disease by limiting the availability of reticulocytes and RBC’s for parasitic invasion but unfortunately may persist worsening anaemia and reducing survival [132]. Parasitaemia, erythropoiesis suppression, and metabolic derangements occur simultaneously showing a common aetiology. Parasite clearance coincides with erythropoietin (EPO) sensitivity and increase in reticulocytosis when inhibiting factor(s) are removed [133-135]. NF-kβ secretion, triggered by malaria infection, modulates the immune response and suppress bone marrow activity through ROS and NO synthesis [84]. The triterpene AA may possibly (through its anti-inflammatory, anti-parasitic, immune-modulation) alleviate malarial disease by inhibiting NF-kβ induction. Moreover, NO inhibits Na+/K+ ATPase which normally protects npRBC’s from premature poor deformability and spleen filterability [136-138]. Anti-inflammatory and anti-oxidant activities of AA may also ameliorate SMA by limiting excessive NO synthesis and availability [82].
Mechanisms of empirical malaria treatment target parasite multiplication termination and transmission. Data in our laboratory has indicated that treatment of intraperitoneal (IP) induced murine malaria parasite P. berghei with AA in SD rats results in dramatic reduction in parasitaemia and SM within 3days concurrently ameliorating metabolic derangements which may suggest simultaneous anti-disease mechanism at play [63].
The absence P. falciparum mature trophozoites and schizonts in peripheral blood smears corroborate the phenomenon of pRBC sequestration. Abrogation of cytoadheresive activity between endothelial cells and RBC seem to be the phenomenon by which patients with sickle cell anaemia and thalassemia have a natural protection against the malarial disease in general and cerebral malaria in particular [139-141]. Cytoadhesion is driven by inflammatory mediators [96,104,142] whose production has been shown to be inhibited by AA in inflammation [33]. Microcirculation obstruction has been advocated as the bedrock of malaria pathophysiology with drugs that show amelioration of circulatory dysfunction vouched to provide efficacious malaria treatment [86,143,144]. Asiaticoside (AS), a glycosylated AA and AA precursor promotes angiogenesis and vascular bed healing [145-148]. In malaria endothelial leakage is the hallmark of vascular damage suggesting that AA may ameliorate malaria sequestration effects [143,149].
Initial cellular adhesion is mediated by cytoadherence receptors ICAM-1 and VCAM-1, glycoproteins expressed on inflammatory mediators-activated lymphocytes, macrophages and vascular endothelium. Pro-inflammatory cytokines, TNF-α, IFN-γ and IL-1β, up-regulate ICAM-1 and VCAM-1 expression [150]. Regulation of ICAM-1 is under the control of NF-kβ system involving ROS [151]. Inhibition of NF-Kβ through reduction of ROS, with subsequent quenching of pro-inflammatory response mechanisms, as seen with AA, may provide mechanisms of reducing cytoadherence. The ligand, PfEMP-1, expressed on pRBC requires different receptors on vascular endothelium in different organs. The triterpenes AA is able to modulate inflammatory responses through NF-Kβ activation inhibition and through activated Th1 cells downregulation by antioxidant and cell apoptotic systems, respectively. These same effects may be applicable to malaria AA treatment with possible selective depletion of activated lymphocytes, macrophages and other cells with aberrant growth patterns in malaria [33,81,152].
Pathogen associated patterns (PAMPS) are naturally detected by local cellular components during malarial infection pre-patent periods resulting in cytokine production with subsequent adhesion molecule production in vascular endothelial cells which facilitates pRBC auto-agglutination, rosetting and vascular occlusion [87,153]. The nuclear receptor superfamily member peroxisome proliferator-activated receptor-γ (PPAR-γ) is expressed in vascular cells and in macrophages where it modulates, together with NF-kβ, the innate immunity epicentre of malaria disease - the vasculature [154-156]. The anti-inflammatory mechanism of AA involves suppression of NF-Kβ activation by way of peroxisome proliferator activated protein γ (PPAR-γ) stimulation in the manner in which NSAIDs binds and activate the nuclear factor [157-160]. Translocation of NF-kβ into the nucleus involves activation of NF-kβ inhibitor (Ikβ) kinase (IKKβ) which phosphorylates Ikβ removing the brakes on NF-kβ for DNA synthesis.
The inhibitory protein Ikβ is maintained in place by activated PPAR-γ thus limiting the activity of NF-Kβ even in the presence of increased levels of its activator TNF-α and globally also through removal of aberrant activated lymphocytes (see above) [161]. AA activation of PPAR-γ consequently down-regulates immune response. By attenuating the cytoadhesion, phagocytic and immunomodulatory activities of local cellular entities, through down-regulation of NF-kβ, AA may induce parasites extrusion when pRBC finally reach the spleen after removal of the anchoring mechanism sequestering them from the organ [151,162,163]. It is not known whether AA has any effect on IKKβ inhibition or whether it can inactivate Ik-β directly without activation of PPAR-γ activation. However, activation of PPAR-γ leads to inhibition of iNOS induction and monocytes inflammatory cytokines meaning that the action of AA on the nuclear ligand may have a wide effect in malaria infection [164-166].
It is not clear whether cytokines influence on cytoadhesive molecules expression also acts on PfEMP-1 expression whose different receptors are found on vascular bed endothelial cells, macrophages and thrombocytes [167]. However, down-regulation of cytoadherence molecules on vascular endothelial cell and up-regulation of CD 36 (influenced by activated PPAR-γ) on monocytes will result in annihilation of vascular occlusion and increased nonopsonic phagocytosis of pRBC’s with concomitant decrease in NF-kβ secretion [93,168]. With cytoadherence having been shown to persist well after effective treatment of P. falciparum malaria AA may provide a mechanism for malaria treatment that might mitigate against continued post treatment intravascular haemolysis [169,171].
There is also a possibility of AA interfering with the parasite’s survival within the red blood cell. The malaria parasite has a very active antioxidant defence system that include glutathione-, thioredoxin-dependent molecules and superoxide dismutase that depletes the oxidative deleterious action of oxidative free haeme [172]. AA may ameliorate oxidative stress in the cell cytoplasm but increase it in the parasite vacuole which has an acidic pH. The triterpene, AA, is able to be both a proton donor and proton acceptor (section 2.1) depending on the environment. Within the Plasmodium food vacuole there is an acid pH, AA may constitutively act as a proton donor and increase oxidative stress that may destroy the parasite. As ROS presents both beneficial and pathological roles, host defence mechanism against the parasite and tissue damage, oxidative stress (OS) holds a promising rationale for AA antimalarial mechanism in the same vein as G-6-PD deficiency confers malaria parasite susceptibility to redox equilibrium [173]. Drugs like quinine and chloroquine, artmisinins have been developed to increase food vacuole OS by either inhibiting haeme to hemozoin biocystralization (aminoquinolenes) or ROS creation (artemisinins) [102,106,174,175]. The redox reaction capability of AA may be involved in antiparasitic activity in the same manner with current drugs while also attenuating cytoadhesion, inflammation and OS.
The mechanisms of action for the pleiotropic phytochemical Asiatic acid in the inflammatory disease like malaria are endless. The fact that the antimalarial activity of the phytochemical have been indicated, albeit in an animal model, raises the possibility of further research opportunities in the areas where biological activities of AA have been shown in other disease with a similar pattern but different aetiology. The anti-inflammatory effect of AA in malaria is an area of great interest that may be explored to elucidate the way in which the phytochemical may modulate systemic mediators and subdue malaria.
Part II of this review seeks to show how AA may influence various systemic aspect of the body in malaria that build up the disease facet of malaria in what is termed anti-disease capacity of AA. (Antimalarial phytochemicals: delineation of the triterpene Asiatic acid malarial anti-disease and pathophysiological remedial activities - Part II).
Acknowledgments go Professor C.T. Musabayane (posthumously) for having pioneered this work and provision of leadership, supervision and unparalleled wisdom. Dr B.N. Mkhwananzi for providing the wisdom and insightful discussion during the formulation of this review. UKZN College of Health Science for part funding of this review (Grant: SN 213574054). National University of Science and Technology-Zimbabwe for part funding of the review.