Barium and Strontium Cobaltites, Synthesized in A Solar Furnace
Perovskite cobaltites of strontium SrCoO3−δ and barium BaCoO3-δ have been studied. It is shown that the technological route, which includes melting a stoichiometric mixture of cobalt oxide with barium or strontium carbonates in a solar fur- nace, quenching the melt into water, grinding the casting and molding, followed by sintering at 11000C, makes it possible to obtain a material based on hexagonal barium and strontium cobaltites with a developed fine microstructure and semicon- ductor properties. the nature of the electrical conductivity.
Keywords:Barium Cobaltites; Strontium; Solar Furnace; Melting; Hardening; Sintering; Ceramics
It is known that perovskite cobaltites of strontium SrCoO3-δ and barium BaCoO3- exhibit a wide range of electronic and magnetic characteristics and are of great interest. A feature of such compounds is the possibility of influencing their transport properties by varying the concentration of anionic vacancies [1]. At the same time, synthesis at high pressures makes it possible to obtain an ideal oxygen stoichiometry (δ=0). For example, SrCoO3 obtained at 6 GPa [2, 3] is a simple cubic perovskite structure.
When SrCoO3-δ oxides are produced at ambient pressure in air, they exhibit the approximate stoichiometry of Sr2Co2O5 (or Sr- CoO2.5). The observed high-temperature brownmillerite-like structures, the so-called “high-temperature phases”, and the hexago- nal structures, called “low-temperature phases” are stabilized due to order-disorder transitions of oxygen vacancies. The complete ordering of vacancies with the formation of the brownmillerite phase is established within a few seconds during quenching after high-temperature (usually 1000°C) solid-phase synthesis [1, 4, 5].
Recently, more and more attention has been paid to barium cobaltite oxide due to its semiconductor characteristics [6-9]. Materials based on BaCoO3-δ doped with some other elements have low resistivity at low temperatures and can be used as thermistors.
In this work, we studied perovskite structures based on barium and strontium cobaltites obtained by melt synthesis in a solar furnace of the corresponding mixture of barium and/or strontium carbonates with cobalt oxide: BCO3 + Co2O3; SrCO3. From the mixture after grinding (63 μm) and molding by semi-dry pressing (P = 1t), samples were made in the form of a cylinder ∅ 20 mm, which were installed on a water-cooled melting unit located on the focal plane of the solar furnace. A concentrated flux of solar radiation with a density of the order of Q=150 W/cm2 was directed to the sample. Such a value of the flux density according to the law of Stefan Boltzmann , where σ=5.67x10-8W/m2K is the Stefan Boltzmann constant, corresponded to the temperature of the heated body of 19000C. At this temperature, the sample melted. Melt droplets fell into water and cooled at a rate of 103 deg/s.In this work, we studied perovskite structures based on barium and strontium cobaltites obtained by melt synthesis in a solar furnace of the corresponding mixture of barium and/or strontium carbonates with cobalt oxide: BCO3 + Co2O3; SrCO3. From the mixture after grinding (63 μm) and molding by semi-dry pressing (P = 1t), samples were made in the form of a cylinder ∅ 20 mm, which were installed on a water-cooled melting unit located on the focal plane of the solar furnace. A concentrated flux of solar radiation with a density of the order of Q=150 W/cm2 was directed to the sample. Such a value of the flux density according to the law of Stefan Boltzmann , where σ=5.67x10-8W/m2K is the Stefan Boltzmann constant, corresponded to the temperature of the heated body of 19000C. At this temperature, the sample melted. Melt droplets fell into water and cooled at a rate of 103 deg/s.
Such cooling conditions made it possible to fix the high-temperature structural states of the material.
Drops of the melt, loaded into the water, cracked into small glass-like particles of arbitrary shape. To study such a material, it was ground to a fineness of 60 μm, dried at 4000C, and samples were molded in the form of cylinders 8mm 15mm high for firing at a temperature of 10000C followed by arbitrary cooling.
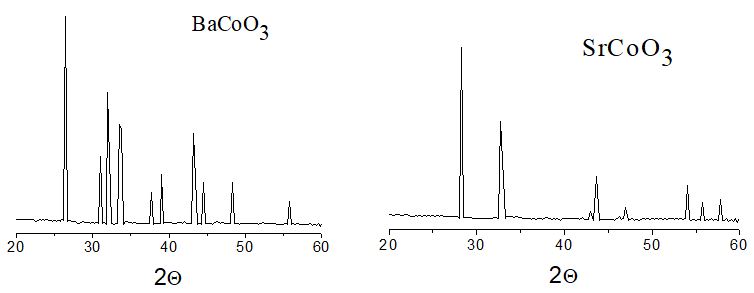
The obtained samples were subjected to X-ray phase analysis using a DRON-3M installation with a copper anode with K-α radia- tion in the Bragg-Brentano reflection geometry with CuKα radiation (λ= 1.5418˚A). The data were obtained between 20≤2θ≤60◦.
The slit system was chosen to ensure that the X-ray beam was completely within the sample over the entire 2θ range.
The temperature coefficient of thermal expansion was measured on a cathetometer in the temperature range 25 - 9500C. The elec- trical resistance was measured by the four-contact method in the temperature range 25 - 10000C.
The density of the samples was determined pycnometrically ,pef=m/vf' the value of which was 4.87g/cm3 for BaCoO3 and 4.64g/cm3 for SrCoO3
The analysis of X-ray patterns showed that for the case of BaCoO3 the diffraction pattern is described by a hexagonal lattice of space group P63/mmc with lattice parameters a=5.652 A, c=4.763A. In the case of strontium cobaltite SrCoO3, a hexagonal structure is also observed with lattice parameters a=9.511A, c=12.287A.
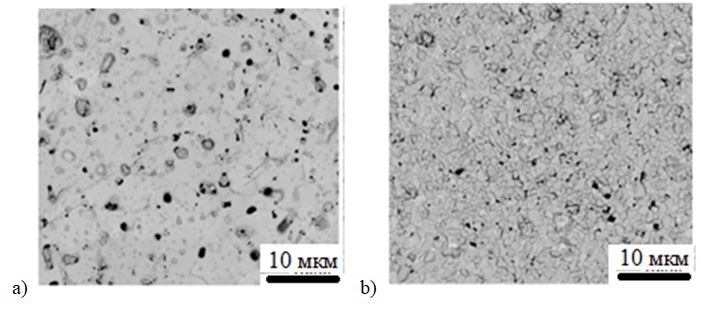
Figure 2 shows SEM micrographs of barium and strontium cobaltites obtained by melt quenching in a solar furnace.
SEM analysis of BaCoO3-δ micrographs shows that the grains have a fine and uniform microstructure. The average ceramic grain size is 3 µm. The relative density of the samples was 94%. The dense microstructure made it possible to obtain good reproducibility of the electrical characteristics of the ceramics.
The temperature coefficient of thermal expansion of the samples in the temperature range 25 - 9500C was α = 11.7x10-6 K–1 for SrCoO3 and α = 14.1x10-6 K–1 for BaCoO3
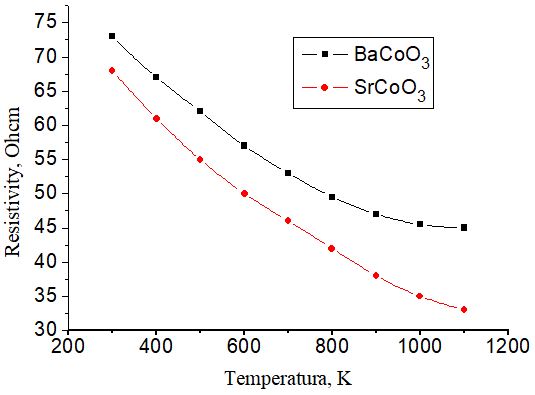
As can be seen from Fig. 3, the resistivity decreases exponentially with increasing temperature. Resistivity depends on temperature and can be expressed by the Arrhenius equation
where ρ and ρ0 are electrical resistivity at a certain temperature and room temperature, respectively. Ea is the activation energy of electrical conductivity. The analysis of the obtained results made it possible to determine the activation energy equal to 0.01 eV. The obtained results indicate that BaCoO3 and CaCoO3 cobaltites, demonstrating high electrical conductivity and low thermal expansion coefficient, can be used as a promising thermoelectric material [10].
Thus, the technological route, which includes melting a stoichiometric mixture of cobalt oxide with barium or strontium carbon- ates in a solar furnace, quenching the melt into water, grinding the casting and molding, followed by sintering at 11000C, makes it possible to obtain a material based on hexagonal barium and strontium cobaltites with a developed fine microstructure and semi- conductor nature of the electrical conductivity. The materials, exhibiting high values of electrical conductivity and low coefficient of thermal expansion, can be used as a promising thermoelectric material.