Basolateral Potassium Channels and Sodium/Potassium Homeostasis. A Fresh Look on Renal Potassium Handling
Potassium channels play a crucial role in renal potassium homeostasis but also in the regulation of blood pressure [1].
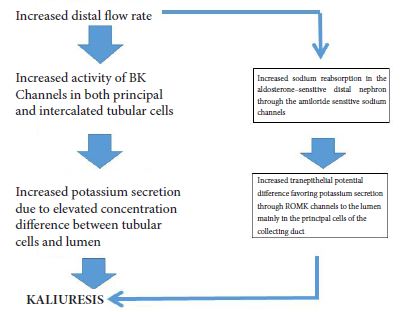
Beyond the ROMK and BK channels located at the apical plasma membrane of the distal nephron which facilitate potassium excretion, basolateral potassium channels in the distal convoluted tubules and collecting duct principal cells seem to play a prominent role in the control of both electrolyte homeostasis and blood pressure [1].
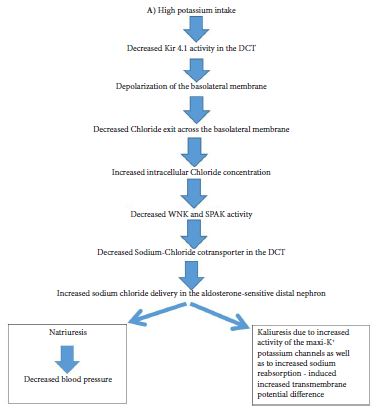
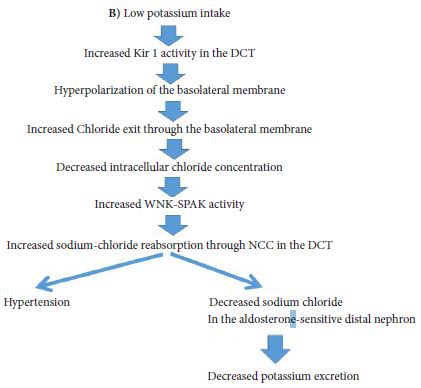
Major interest has focused on both Kir 4.1 and Kir 5.1 channels being a key-determinant of the resting membrane potential of both distal convoluted tubules and collecting duct tubular cells [2-4]. In fact, mutations in KCNJ 10 gene which encodes the Kir 4.1 channels is associated with the SeSAME EAST syndrome, characterized by seizures, salt wasting, epilepsy, mental retardation, ataxia, and sensorineural deafness, while mutations in the KCNJIβ gene encoding the kir.51channels is associated with the nonfamilial Brugada syndrome associated with sudden cardiac death as well as with hypokalemia associated with hyperchloremic metabolic acidosis and hypercalciuria [2]. Recently data suggest that the kir 4.1 channel in the distal convoluted tubules plays a cardinal role in potassium homeostasis but also in the regulation of sodium homeostasis and blood pressure [4]. In fact, recently published data confirm that Kir 4.1 channels act as a sensor of dietary potassium intake and mediates the activity of the thiazide-sensitive sodium-chloride cotransporter (NCC) in the distal convoluted tubules thus affecting sodium chloride delivery in the aldosterone sensitive distal nephron resulting in both changes in potassium and sodium excretion and subsequently in the regulation of blood pressure [5]. The Kir 4.1 channels recycle K+ ions across the basolateral membrane which moved into cells through the K+-Na+-ATPase leading to a negative basolateral membrane potential that plays a paramount role in sodium reabsorption and potassium secretion through changes in the NCC activity [5]. Furthermore, it should be emphasized that NCC activity affects both sodium and potassium excretion; thus high activity of NCC is associated with familial hyperkalemic hypertension (also called pseudoaldosteronism type II), while in Gitelman syndrome mutations in the gene encoding NCC is associated with hypokalemia and hypomagnesemia and low blood pressure [5].
The role of Kir 4.1 channels in both sodium and potassium homeostasis is depicted in Figure 1A & Figure 1B and Figure 2. Thus, the activity of Kir 4.1 channels which is affected by oral potassium intake results in changes in polarization of the basolateral membrane and subsequently affects intracellular chloride concentrations [5]. These changes of intracellular chloride concentrations can subsequently affect the NCC activity through changes in the activity of the so called with no lysine kinases (WNK) as well as ste20 proline-alanine-rick kinase (SPAK) pathway [5].
These data explain the clinical and experimental data which have suggested that dietary supplementation of potassium results in a decrease in blood pressure in both human and animal models, especially in cases of salt sensitive hypertension [1,5]. The high potassium diet induced elevation of aldosterone significantly contributes to the increased kaliuresis and the resultant restoration of potassium balance [5]. Extrarenal mechanisms may also play a role in the final regulation of potassium homeostasis through the gut [1].