Biochemical Characterization and Biological Evaluation of Royal Jelly from Apis cerana
Royal Jelly (RJ) is an extremely nutritious functional food produced by nurse honey bees from their hypo- pharyngeal gland. It plays a critical role in the determination of honey bee caste and the development of queen bee. RJ is also considered as a ‘nutraceutical’ often used as a substance that supports health. The present study analyzes the biochemical components of Royal Jelly from Indian honey bee, A. cerana. RJ showed an acidic pH 3.6. Royal Jelly has a higher moisture content of 67% and ash content of 1.06%. RJ has the lipid and the carbohydrate content of 4.6 mg/mL and 11.4 mg/ml respectively. This highlights the properties of RJ in various fields of medicine, food and cosmetic industries. Antimicrobial activities were also evaluated and were found to be effective against Bacillus subtilis and Rhizopus stolonifer. It also showed high antioxidant potential which shows its ability to scavenge free radicals thus protects the cells from unwanted stresses. This paper reports for the first time the biochemical characterization of Royal Jelly from Indian honey bee, A. cerana.
Keywords: Royal Jelly; Functional food; Antimicrobial activity; Phenolic compounds; Flavonoids contents; Antioxidant activity
Royal Jelly (RJ) is a thick milky white secretion produced from the hypo pharyngeal glands of nurse honey bees of 6-12 days old worker bees. It plays an important role in the caste determination and the development of the queen bee. The larvae destined to be queen bees are continuously fed with RJ but the larvae destined to be worker bees fed with a mixture of honey, RJ and pollen [1]. RJ has been discovered to have anti-ageing, antibacterial, anti-oxidant, anti-tumor, ant fatigue, anti-inflammatory, vasodilative, hypotensive, disinfectant, anti-hypercholesterolemia, anti-diabetic properties [2,3], anti-mutagenic and anti-histopathology effects [4]. These effects are attributed to the bioactive components present in the RJ. It includes water (60-70%), protein (12-18%), sugars (8-11%), lipids (3-8%) and vitamins, salt and free amino acids. There are differences in composition of RJ according to seasonal and geographical conditions, species of honeybees [5], physiological and metabolic changes of both nurse bees and larvae [6], time of harvest of RJ [7]. The objective of the study is to analyze the biochemical characterization and biological properties of RJ from Indian honeybee, A. cerana. This is the first report showing the biological characterization of RJ from Indian honey bee, A. cerana
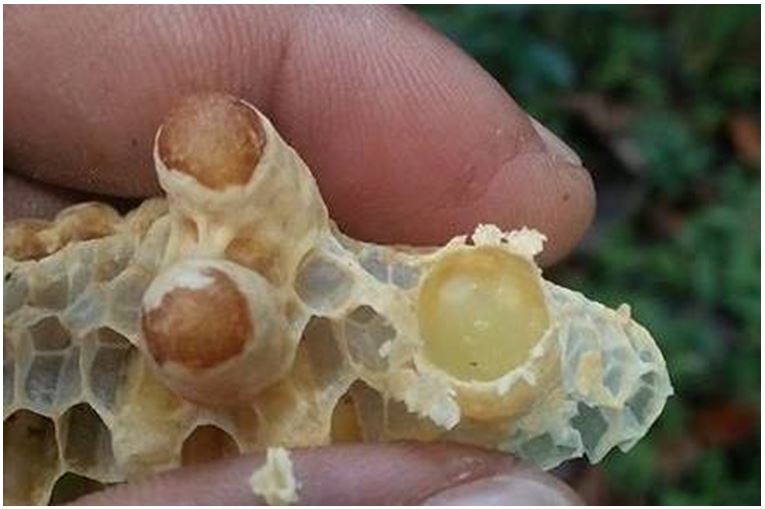
RJ was collected in January from the queen cells at the Apiary of Meenachil Bee Garden, Pala, Kottayam, Kerala, India. The queen cells containing the RJ was removed from the frame and immediately placed in -20 °C wrapped with aluminum foil. The samples were thick milky in appearance, slightly yellow in color (Figure 1) with a pungent odour. Before use, the stored samples were allowed to equilibrate at room temperature and stirred to produce a homogenous mixture.
An investigation on the biochemical composition of RJ was carried out as follows. International Honey Commission (IHC) by Sabatini, et al. developed an international standard for RJ [8].
Determination of pH [9]: pH of RJ was determined by using a pH meter.
Determination of moisture content [9]: RJ (5.0 g) was placed in a hot air oven for eight hours at 105 °C in pre-weighed crucibles.
The crucibles were periodically weighed and the weights recorded Equation 1.Triplicates were maintained to reduce errors in generating data. The reduction in weight represented the moisture content of the samples.
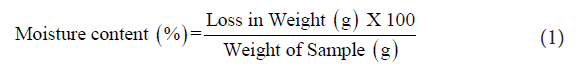
Ash content [9]: The sample was weighed to 5 g in pre-weighed crucibles, charred on a hot plate and then placed in a muffle furnace at 600 °C for 4 h. From the weight of residue left in the crucible, the total ash content was calculated Equation 2.
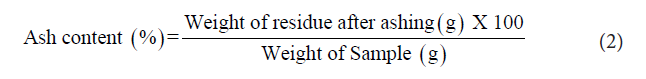
Total carbohydrate content: The quantity of sugar in RJ was estimated by phenol sulphuric acid method suggested by Dubois, et al. [10]. 1 mg sample was diluted in water (1:1) and then 1 mL of 80% phenol and 5 mL of 96% w/v sulphuric acid solution were added in to it. The concentrated sulphuric acid breakdown sugars to monosaccharaides which are then dehydrated to form furfural and their derivatives. These compounds then react with phenol to develop a yellow orange color. Fructose (1 mg/mL) was used as the standard. The samples were read at a wavelength of 490 nm in a UV/VIS spectrophotometer (Shimadzu) when the range colors develop.
Determination of lipid content: Lipid content of the sample was estimated by sulpho phosphovanilin method [11]. 2 ml of concentrated sulphuric acid was added to a test tube containing the sample (1 mg). The mixture was heated for 10 minutes in a boiling water bath and then cooled for 5 minutes. Phosphovanillin reagent was added to each tube, mixed well and incubated at 37 °C for 15 minutes until the solution turns pink where intensity depends on the quantity of lipids present. The absorbance was read at 540 nm. A graph was plotted with olive oil (1g/100 mL absolute ethanol) as the standard.
Estimation of protein: Total protein was estimated by Lowry’s, et al. method [12]. The assay is based on the biuret reaction of proteins with cupric sulphate at alkaline conditions and the Folin-Ciocalteau phosphomolybdo tungustate reduction to heteropolymolybdenum blue by the copper-catalyzed oxidation of aromatic acids which is measured at 660 nm.
To 1 ml of sample, 5 ml of Alkaline copper sulphate was added and mixed well. The whole contents were incubated at RT for 10 minutes. 0.5 ml of Folins Ciocalteau reagent was added and kept at dark for 30 minutes. 2 ml of NaOH (0.1 N) serves as blank. The resulting colour was measured at 660 nm. Bovine serum albumin was used as standard which was prepared in a concentration of 1 mg/ml. Concentration of the protein is estimated by comparing the test values against standard curve.
Anti-fungal activity of RJ: Anti-fungal activity of the RJ samples was studied using various fungal stock cultures of Aspergillus niger, Aspergillus flavus, Rhizopus stolonifer and Candida albicans. Stock culture of the fungus was maintained at 4 °C on Sabouraud dextrose agar. The stock culture was sub cultured in sarbourod dextrose broth (SDA) and allowed to grow for 3 days. In agar well diffusion method, wells were cut using sterile well bore of 6 mm diameter and 0.1 ml of each of the prepared culture of test fungus was loaded on the agar plate. The plates were swabbed uniformly with a sterile swab and allowed to dry for 5 minutes. Candid (1 mg/ml) was used as positive control whereas deionized water was used as negative control. Two different concentrations of RJ samples (50μl and 100μl) transferred to the wells and the antifungal activities were observed after incubating the plate after 72 hours of incubation at 37 °C. Zone of inhibition was also measured after incubation.
Anti-bacterial activity of RJ: Anti-bacterial Activities of the RJ samples were studied by agar well diffusion method against various bacterial cultures of Escherichia coli (MTCC40), Staphylococcus aureus (MTCC 3160), Bacillus subtilis (MTCC 121), and Pseudomonas aeruginosa (MTCC 422), Klebsiella pneumoniae (MTCC 403) and Salmonella typhimurium (MTCC 98). Organisms were allowed to grow in Mueller Hinton Agar (MHA) medium. Agar wells were cut using sterile well bore of 6 mm diameter and 0.1 ml of each of the prepared culture of test bacterial cultures was loaded on the agar plate. The plates were swabbed uniformly with a sterile swab and allowed to dry for 5 minutes. Two different concentrations (50μl and 100μl) of RJ samples of 1 mg/ml transferred to the wells and the antibacterial activities were observed after incubating the plate for 24 hours at 37 °C. Antibacterial activity was evaluated by measuring the diameter of the inhibition zone. Here streptomycin (2 mg/ml) was used as positive control and deionized water was used as negative control.
Determination of total phenolic content: Total phenolic acid content of RJ was determined by Folin-Ciocalteu method according to Singleton, et al. with slight modification [13]. The basic mechanism is an oxidation/ reduction reaction. In this carbonate buffer is used for the pH adjustment and the end point of the reaction was attained after 2 hours at room temperature. 0.5 ml of the RJ samples was added to 2.5 ml of 0.2 N Folin- Ciocalteu reagents. The mixture was incubated for 5 minutes. Four ml of the 20% of sodium carbonate were added and incubated in room temperature for 2 hours and absorbance was measured in 760 nm. Gallic acid (0.01-0.05 mg/ml) was used as standard at the concentration of 0 to 1 ml. Absorbance was measured in 760 nm and methanol was used as blank. Total content of phenols was expressed in milligrams of gallic acid equivalents (mgGaA. 100 G-1).
Determination of flavonoid content:The total flavonoid content in RJ sample was measured using the assay developed by Zhishen, et al. [14]. 2 g of RJ was dissolved in 20 ml of deionized water and filtered using whatman grade 1 filter paper. To that 0.3 ml of sodium nitrate solution (5g/l) was added. After five minutes of incubation, 0.3 ml of AlCl3 (10% w/v) were mixed to RJ. 2 ml of NaOH (1 M) was added followed by it and kept it on 6 minutes. The volume was increased by the addition of 2.4 ml distilled water to 10 ml. The mixture was shaken and the absorbance was read at 510 nm. Quercetin was used as standard and the values were expressed in milligrams of quercetin equivalents (mgQE.100 G-1).
Total anti-oxidant activity of RJ using DPPH: The scavenging activity of honey samples against DPPH radical was analysed according to the method of Ferreira, et al. [15]. The assay mixture contained 0.5 ml of RJ and 2.7 ml of methanolic solution containing DPPH radicals (0.024 mg/ml). The mixture was shaken vigorously on a vortex mixer then incubated 90 minutes at 25 °C in a water bath in the dark, after which the absorbance was determined at 517 nm against blank. Blank was take as all the reagents in same concentration except DPPH. The scavenging activity was expressed as IC50 (mg/ml). Ascorbic acid was used as standard. The Radical Scavenging Activity (RSA) was calculated as % RSA= ([ADPPH-AS]/A DPPH)* 100), whereas as is the absorbance of the solution and ADPPH is the absorbance of the ADPPH solution. All the assays were performed in triplicate.
RJ is a viscous jelly substance, often not homogenous due to the presence of un-dissolved granules of varying size. It is partially soluble in water and slightly acidic with a high buffering capacity. Moisture, ash, sugars, lipid and protein contents were also studied using biochemical assays and these can be used as criteria for checking the quality of RJ.
Although the chemical composition of RJ is relatively constant, there could be significant variations in each constituent in samples from different regions [7]. The results obtained agree with the report by Balkanska [16], in RJ from Bulgaria and by Food and Agriculture Organizations [17].
pH:pH is an important parameter for assessing the quality of RJ sample. The pH value of RJ was measured and confirmed to be acidic at pH 3.6 and lies within the standard limit of pH 3.4-4.5.
Moisture content: RJ had a water content of 67% which agreed with the standard limit of 60-70%. This parameter is an important quality criterion of raw RJ.
The water content in RJ was found to be similar to that found in the previous reports of Jie, et al. which was 50.2% and 67.2% [18]. Harvesting period also affects the moisture content in sample. The moisture content changes when harvesting of RJ is delayed. Kanelis, et al. suggested that delayed collection of RJ gives its very low water content [19]. The moisture content may also increase at the time of transfer. Here the moisture content of sample was higher which may either increase according to the collection time or due to time of transfer. The present sample was collected from the first day old queen cell from the hive.
Ash content: Percentage of ash in RJ was 1.06% and it is comparable to the standard limit of 0.8-3%. The ash content is a measure of the total amount of minerals present within RJ. The ash content of RJ examined was agreed with the previous observation by Krell suggesting that fresh and dry weight of RJ was found to be 1% to 3% respectively [17].
Total carbohydrate content: Total carbohydrate present in RJ was 11.4 mg/ml. This value was observed to be within the acceptable range of 7-18%.
The amount of sugar present in RJ is relatively similar to the previous report by Nabas, et al. [20], which was also within the limits proposed by Sabatini, et al. [8]. The total sugar content was mostly occupied by glucose and fructose [21]. The content of sugar depends on the plant source but didn’t show that much variation between samples from different regions [22]. Popescu, et al. proved that sucrose in sugar varies considerably from one sample to another [23].
Total lipid content: The total lipid content in RJ was 4.6 mg/ml and it is in the acceptable range of 3-8%. It is the third most important component of RJ after proteins and carbohydrates.
In the study of Sabatini, et al. lipid content of RJ was 8-19% of the dry matter [8]. The value obtained in this study is also within that range. The lipid fraction consists of fatty acids, waxes, steroids and phospholipids. The major one is 10-hydroxy-2-decenoic acids (10-HDA) which vary depending on the season and the region. Among the lipid fractions, HDA is well known renowned for many medicinal properties [24,25]. It is also used as freshness marker to check for the adulterants in RJ [21]. It was thus concluded that RJ sample of A. cerana from Indian honey bee is of good quality and can meet International standards.
Estimation of protein by Lowry’s method: The total protein content of RJ was estimated to be 12.5 mg/ml.
Proteins are the second most abundant components in RJ after water which plays an important role in queen bee differentiation. The obtained value was closest to the findings of Balkanska and Zhelyaskoa in RJ from Bulgaria of 14.7 mg/g [26].
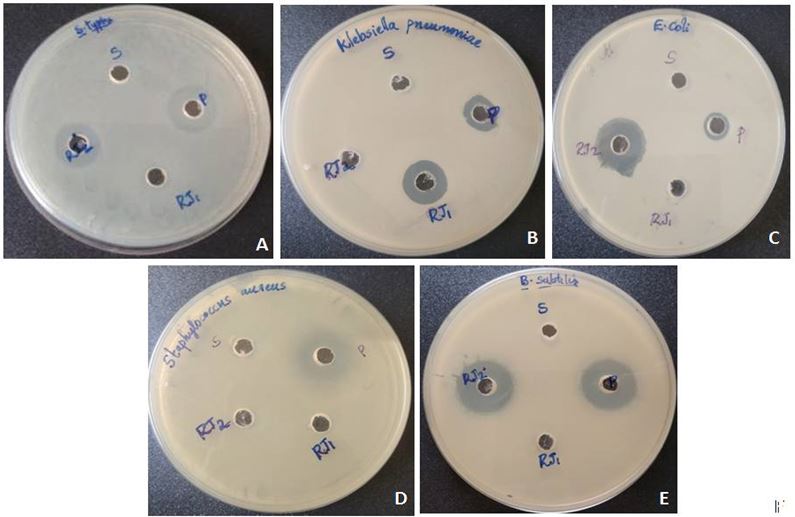
Anti bacterial activity of RJ: The inhibitory action of RJ on the growth of bacteria is shown in Figure 2. RJ showed maximum antibacterial activity against B. subtilis at a concentration of 100 μg/ml with zone of inhibition of 25 mm. Other strains such as E. coli and S. tyhphimurium showed the zones of inhibition of 22 and 19 mm respectively at the same concentration of 100 μg/ml. K. pneumoniae showed inhibitory activity at the concentration of 50 μg/ml. RJ showed no activity against S. aureus at both concentrations.
In the present study RJ exhibited both antibacterial and antifungal activity against the organisms tested. The highest antibacterial activity was observed against B. subtilis at a concentration of 100 μg/ml. The present results indicated that RJ is more effective against Gram positive microorganisms. The results also agreed with the studies of Fujiwara, et al. who report that royalisin, an antibacterial peptide showed antibacterial activity against Gram positive bacteria [27]. Moselhy, et al. and Bilikova, et al. also demonstrated the effect of RJ against Gram positive organism, B. subtilis [28,29]. On the contrary the result also showed that RJ did not have any antimicrobial activity against another Gram positive microorganism, S. aureus at both concentrations. According to Blum, et al. the bactericidal activity of RJ is due to the presence of 10-HDA present in it [30]. This variation of antibacterial activity of RJ samples depends on many factors including its geographical origin and the honey bee species.
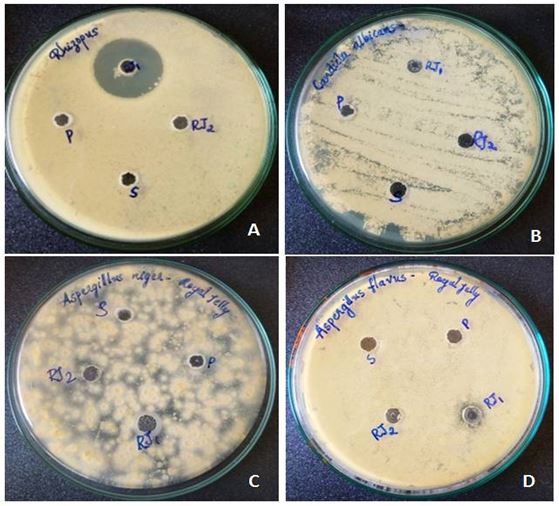
Antifungal activity of RJ: RJ showed maximum antifungal activity against R. stolonifer among the fungal strains. The average diameter of the inhibition zone produced by the RJ samples was 25 mm at a concentration of 100μg/ml. No activities were observed against A.niger, A. flavus and C. albicans at both concentrations (Figure 3).
RJ had the highest antifungal activity against R. stolonifer at an extremely low concentration of 50 μg/ml. No inhibitory activity was observed against A. flavus, A. niger and C. albicans.
Total Phenolic acid content: The phenolic content of the RJ sample was estimated to be 2.55 g/ dry matter.
The present results of phenolics content in RJ agreed with the previous studies of Nabas, et al. from A. mellifera species [20]. They reported that RJ contains 23.3±.92 μg/mg of phenolic compounds. Similar results were reported by Balkanska, et al. [32] of 11.66-36.73 mgGAE/g in Bulgarian RJ and 59.16±5.92 mg GAE/100g in Turkish RJ [33]. Polyphenolic compounds in RJ were higher when it was harvested within 24 hours after larval collection when compared with 48 or 72 h [35].
Total Flavonoid content: The flavonoid content of RJ was estimated to be 0.5 g/ dry matter.
The flavonoid content of RJ was similar to the value, ±0.09 RE μg/mg of flavonoid which was obtained by Nabas, et al. from A. mellifera species [20]. Both phenolics and flavonoid compounds present in RJ provide antioxidant activity to
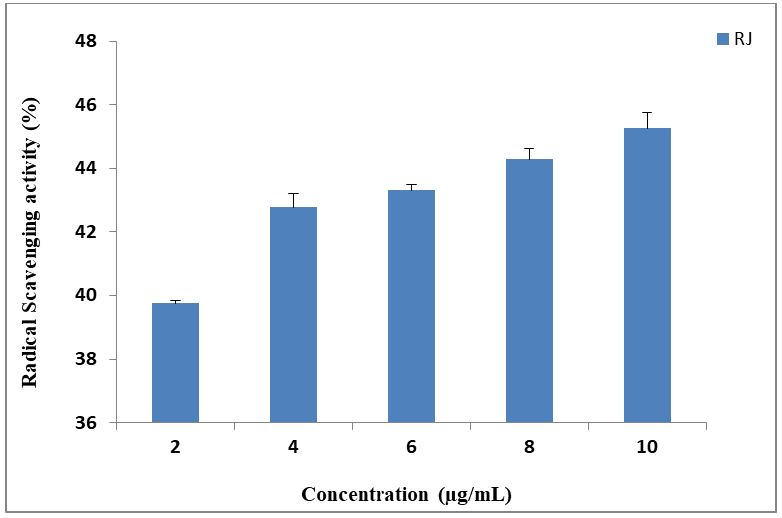
Total Antioxidant assay using DPPH: The results showed that RJ samples has a lower scavenging activity (45.7%) (Figure 4) when compared to that of ascorbic acid (91.4% in 10 μg/ml). The IC50 value of RJ sample was found to be 14.4 μg/ml as compared to 2.15 μg/ml in ascorbic acid.Several reports are available about the antioxidant activities of RJ. Liu, et al. reported that DPPH radical scavenging activity of RJ was 43.0-62.8% and 10.17 to 39.39% in Bulgarian RJ sample [32]. Balkanska, et al. attributes the antioxidant activity of RJ to the presence of organic acids and higher polyphenolic content in RJ [32]. Antioxidant action of phenolic compounds has higher tendency to chelate iron and copper. This is because of the presence of hydroxyl and carboxyl groups of phenolic compounds. They inactivate iron ions by chelating and additionally suppressing the superoxide driven fenton reaction, which is the most important source of ROS [35].
To sum up, following are the findings of the present study. The results suggested that RJ can be considered not only for its nutritive value but also a possible resource to increase the natural defense of the organism against pathogenic microorganisms. Thus RJ owing to its immense nutritive value can be utilized as functional ingredients in foods too
Funding for this work was provided by UGC, New Delhi. We also thank Meenachil Bee Garden, Pala, Kottayam for providing Royal Jelly from A. cerana for our studies.