Circulating MicroRNAs as Potential Biomarkers for Prostate Cancer
Prostate cancer (PCa) is the most dominant cancer among men in America and Europe. Currently used clinical diagnostic markers are ineffective due to low specificity and poor sensitivity. A novel biomarker for diagnosis of PCa is required. A large quantity of microRNAs (miRNAs) is built up of 18-23 nucleotides; they are small non-coding and single-stranded, and are important in post-transcriptional regulation of gene expression by degrading or suppressing target gene mRNAs. MiRNAs are implicated in the pathogenesis of prostate cancer; however, they also act as novel target for the therapeutic intervention. They also show promise as biomarkers. In this review, we discuss the role of circulating miRNAs as potential biomarkers for PCa diagnosis.
Keywords: MicroRNAs (miRNAs); Biomarker; Diagnosis; Prostate Cancer; Post transcriptionalREVIEW
Prostate cancer (PCa) is the most commonly diagnosed malignancy and leading cause of cancer mortality in men worldwide. The American Cancer Society reported that there will be 180,890 new cases of PCa and 26,120 deaths in 2016 [1,2]. PCa development is a slow process commonly in men over the age of fifty years and mainly develops at peripheral zone and involves multiple genetic and molecular changes [3,4]. Prostate specific antigen (PSA) is the only gold standard diagnostic biomarker and indicator for the PCa management. Inclusion of PSA screening into clinical examinations has achieved a significant reduction in PCa-associated death. Although in most of the cases, PSA testing is not disease-specific. As a result, PSA periodical screening led clinicians to misdiagnose and overtreatment the disease and also performed unnecessary prostate biopsies in order to identify a patient with a lethal prostate tumor. This happened due to low specificity and sensitivity as the PSA levels were elevated even in benign prostatic hyperplasia (BPH), chronic inflammation and infection [4-7]. Due to these shortcomings, detection of PCa is not effective. Other biomarkers have been proposed such as total PSA velocity (total PSAV), human glandular kallikrein 2 (hk2), urokinase plasmonogen activator (uPA), urokinase plasmonogen receptor (uPAR), transforming growth factor-beta 1 (TGF-β 1), interleukin-6 (IL-6) and interleukin-6 receptor (IL-2R) for diagnosis [5,6]. However, developing novel biomarkers to detect with accuracy would greatly assist the diagnosis of PCa.
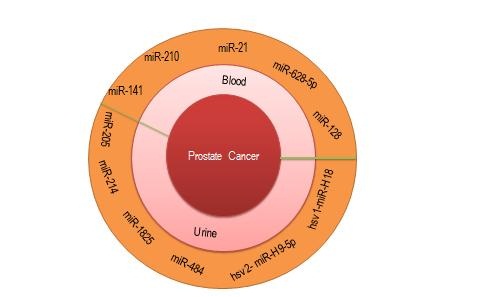
The importance of microRNAs has been established in different types of cancer including prostate cancer. A large quantity of microRNAs (miRNAs) are built up of 18-23 nucleotides; they are small, non-coding and single-stranded and are important in post-transcriptional regulation of gene expression by degrading or suppressing target gene mRNAs. MiRNAs are active by binding to the 3ʹ-untranslated region of various cancer gene mRNAs. Human encoded genes are composed of 3% of miRNAs and above 30% of mRNAs are regulated by miRNAs which affect various physiological and pathological mechanisms including cell cycle, cell differentiation, cell growth, immune response, metabolism, apoptosis and metastasis. Moreover miRNAs are involved in neuronal, muscle, germline development and embryonic morphogenesis and also participate in stem cell regulation [5,8-10]. Recent studies have identified miRNAs from various sources such as prostate cell lines, prostate tissue, urine, blood, prostate cancer xenograft, and benign prostatic hyperplasia (BPH) [11,12]. The use of circulating miRNAs as potential non-invasive biomarkers for Pca could become promising (Figure 1).
A large number of miRNAs has been linked to different cellular pathways such as apoptosis, cell proliferation, metastasis and metabolism. About 30% of genes regulation is influenced by miRNAs and 50% of miRNAs are identified within introns of a gene ( Table 1) [8,21].
MiRNA-34a is downregulated and functions as tumor suppressor in PCa. Induced MiR-34a expression inhibits cell proliferation, invasion and promotes anti-apoptotic proteins. Ras oncogene is involved in the development and progression of prostate cancer. The effectors are shown to have potential as therapeutic targets for the treatment of androgen-independent prostate cancer. An abnormal WNT/β-catenin signaling is found to be involved in several types of cancers including PCa. The combined activation of Ras and WNT oncogenes leads to the rapid progression of invasive carcinoma from tumorigenesis. MiR-34a is reduced by Ras signaling and functions as a negative regulator of WNT signaling by directly targeting the 3ʹ-UTR of TCF7. Losing of MiR-34a expression leads to an oncogenic effect on cell growth and invasion and in Ras signaling-activated prostate cancer cells [22,23]. In addition, PCa stem cells enhanced clonogenic, tumor-initiating and metastatic capacities; they were enriched in the CD44+ cell population. Enforced expression of MiR-34a in bulk or purified CD44+ prostate cancer cells inhibited clonogenic expansion, tumor regeneration, and metastasis. This revealed that MiR-34a is a key negative regulator of CD44+ prostate cancer cells and showed that CD44 is a direct functional target of MiR-34a to inhibit prostate cancer regeneration and metastasis [24].
MicroRNA MiR-19a is upregulated in castration-resistant prostate cancer (CRPC) and functions as oncogene. The inhibition of MiR-19a overexpression in CRPC cells suppressed proliferation and regulated apoptosis. The increased BTG1 expression in CRPC cells also significantly suppressed cell growth and regulated apoptosis. B-cell translocation gene 1 (BTG1) is a family of BTG nuclear protein and it has highest expression in the G0/G1 phases of the cell cycle and is decreased when cells progress through G1 which can inhibit cell proliferation, metastasis, angiogenesis and regulate cell cycle progression and differentiation in various cell types. The study revealed that MiR-19a regulates proliferation and apoptosis of CRPC cells by directly targeting the tumor suppressor gene BTG1 [25-27].
MicroRNA MiR-154 is downregulated in prostate cancer. Restoration of MiR-154 decreased the potential of prostate cancer cells to grow and proliferate and also down-regulated the expression of Cyclin D2 (CCND2) by binding to 3’-untranslated region. Cyclin Ds (CCNDs) are regulators of cell cycle progression and function as transcriptional co-regulator. The aberrant expression of CCND2 leads to abnormal cells proliferation. Thus, MiR-154 plays an important role in PCa proliferation by suppressing CCND2. In another study MiR-154 was regulating epithelial mesenchymal transition (EMT) by targeting high-mobility group AT-hook 2 (HMGA2). EMT contributes in the process of invasion and metastasis of human cancers and HMGA2 is also involved in the EMT process by aberrant expression in malignant tumors. Forced expressions of MiR-154 or HMGA2 reduced the migration and invasion abilities of PCa cells and inhibited EMT [28,29].
Autophagy plays a potential role in cancer development and cancer cell survival modulating multiple tumor hallmarks. It serves as a protective process to prevent cancer initiation and promote tumors cell survival and maintenance after neoplastic transformation. Angiogenesis and senescence play vital roles in tumour development. Sirtuin (SIRT) is highly conserved family of lysine modifying nicotinamide adenine dinucleotide (NAD+)-dependent class III histone deacetylases; it regulates the signaling pathways by targeting expression of genes involved. SIRT1 is highly expressed in PCa. SIRT1 regulates p300/calcium binding protein, androgen receptor and cell cycle proteins like Rb which are involved in regulating physiological processes such as proliferation, metabolism, differentiation, survival, energy homeostasis, aging and pathological conditions. MiR-212 is down regulated in prostate cancer and plays a vital role in PCa development. Loss of MiR-212 in PCa could lead to induction of autophagy for tumor promoting cell survival and aggressive disease. The negative regulation of MiR-212 in PCa leads to modulation of autophagy, angiogenesis and cellular senescence. SIRT1 modulates autophagy and angiogenesis and also contributes to influencing life span for calorie restriction and senescence in tumor cell growth. Therefore, MiR-212 negatively modulates starvation induced autophagy in PCa cells by targeting SIRT1 and overexpression inhibits angiogenesis and cellular senescence [14,30-32].
Nuclear factor-kappaB (NF-kB) is a transcriptional factor and plays important roles in various biological processes. Aberrant activation of NF-kB is involved in the progression and also in metastasis and invasion of PCa through matrix metalloproteinase-9 (MMP-9). In a normal pathway, inhibitors of NF-kB (IkBs) kinase â (IKKâ) activate NF-kB by phosphorylation of IkBs. MiR-497, involved in prostate cancer, functions as tumor suppressor and is downregulated in PCa. The aberrant expression of MiR-497 changed cellular proliferation, migration and invasion in PCa cell line PC3-AR by directly targeting IKKâ. The proliferation was suppressed by inducing G0/G1 cell cycle arrest and decreasing of CDK8 protein level after transfection of MiR- 497. Thus, MiR-497 repressed the expression of IKKâ and downregulated the activity of CDK8 in PCa [33].
Urine is one of the most easily accessible and non-invasive biofluids available in urology, nephrology and primary care clinics. The use of a urine RNA-based evaluation appears to be fraught with problems given the known liability of RNA; however, there is increasing evidence that miRNA shows surprising stability in situations in which total RNA or mRNA have been shown to be degraded. Mall et al. studied the urinary miRNA stability under various clinically relevant conditions: room temperature and 4 oC, as well as serial freeze–thaw conditions. They revealed that miRNA is relatively stable in the harsh urinary environment; even storing for 5 days at varied temperatures and after ten freeze–thaw cycles and even after trypsin digestion; despite modest degradation, there remained sufficient miRNA for quantitative analysis and did not alter miRNA stability. Further, they suggested that urinary miRNA is best measured within the first 24 h for the most accurate representation of miRNAs in the urine milieu. Moreover, the different miRNAs have shown similar stability when evaluated in plasma and serum [34-36].
MiRNAs that are identified in urine could play an important role as molecular diagnostic markers having non-invasive diagnostic potential. It will overcome the physiological and anatomical problems. In one of the urinary miRNA-based study from 73 urine samples patients with diagnosis of PCa having ≥7 Gleason score and 70 patients diagnosed with BPH revealed that miRNAs are stable molecules which can be measured in urine. This study demonstrated that the elevation of MiR-100 and MiR-200b levels in urine samples was significantly associated with the presence of advanced PCa; moreover MiR-100 was found up-regulated in high-grade prostate intraepithelial neoplasia and also was up-regulated in sera from patients with metastatic castration resistant prostate cancer (mCRPC). Thus, MiR-100/200b could be significant non-invasive biomarker for PCa [4,37,38].
MiR-205 is downregulated and is shown to epigenetically repress tumour suppressor in PCa. It is also reported that MiR-205 is a target of BCL-2 gene, Androgen receptor (AR), and protein kinase C epsilon. The loss of MiR-205 function is associated with low prognosis, apoptosis resistance in PCa and hallmark of epithelial-mesenchymal transition, which lead to metastasis [10,13,33,39-45]. MiR-214 is expressed aberrantly and is downregulated in PCa Urinary MiR-205 and MiR-214 level together could discriminate PCa patients from healthy individuals with 89% sensitivity and 80% specificity, suggesting that this miRNAs could provide non-invasive molecular biomarkers for detection of PCa [11].
MiR-1825 is upregulated in 88% of PCa patients and has putative targets in member-1 of the Discoidin Domain family of receptors (DDR1). MiR-484 is downregulated in 75% of PCa patients and functions as tumor suppressor; it regulates the expression of E3 ubiquitin-protein ligase (UBR5). MiR-1825 diagnosed PCa by 60% sensitivity and 69% specificity while MiR-484 diagnosed PCa has 80% sensitivity and 10% specificity. The combination of MiR-1825/484 could diagnose PCa by 45% sensitivity and 75% specificity [46,47]. Urine-circulating MiR-21 was significantly in lower levels in PCa patients. In PCa tissues, elevated levels of MiR-21 have been detected which showed significant associations with PCa progression. A higher level of serum MiR-21 was found in both androgen-dependent PCa (ADPC) and hormone-refractory PCa (HRPC) patients with low PSA levels. Moreover, other studies showed the associations between blood MiR-21 levels and aggressive course of PCa. Therefore, the difference of MiR-21 levels in urine and blood can be explained by specific miRNA regulation in separate body compartments and selective secretion of this miRNA into body fluids. MiR-21 plays a crucial role in prostate carcinogenesis by promoting cell proliferation, inhibiting apoptosis, enhancing tumor invasion and metastasis; this miRNA could be considered as a PCa-specific biomarker accessible by lowly invasive ways in urine or blood [48-54]. Therefore, urinary miRNAs could assist in prognosis, diagnosis and prediction of PCa.
Association of circulating miRNAs in PCa had established their possibility to be developed as molecular biomarkers for PCa. Most of the blood based biomarkers are useful for prognosis, diagnosis and effective treatments. One of the pioneering research groups revealed the importance of MiR-141 as circulating biomarker from serum. The authors studied 6 miRNAs from 25 metastatic prostate cancer patients and 25 healthy men. MiR-141 was overexpressed in the PCa when compared to healthy control; MiR-141 showed the greatest differential expression among the other miRNAs. Another study analyzed the expression of 667 miRNAs on serum from advanced PCa patients. In this study MiR-141 and MiR-375 expressions were enhanced in PCa samples and their release into the blood was associated with advanced PCa as compared to the other miRNAs. Therefore, MiR-141 had a sensitivity of 78.9% and specificity of 68.8% in predicting clinical progression (17,34,55,56). Hence MiR-141 could also be used as a biomarker.
A study of cancer associated 365 miRNAs which were differentially expressed in serum of metastasis castration resistant PCa and a healthy sample was carried out. Out 365 miRNAs, five serum miRNAs (MiR-141, 200c, 200a, and 375) were increased when compared to healthy 210 samples. The expression of plasma MiR-210 had been reported to be increased in pancreatic patients and was found to be indicator of hypoxia; moreover MiR-210 targeted mTOR in PCa which lead to AKT activation and HIP-1 alpha transcriptional activation. This study revealed that serum MiR-210 levels varied widely amongst mCRPC patients undergoing therapy and showed correlation with the treatment response as assessed by changes in PSA. This indicated that serum MiR-210 can be developed as a predictive molecular biomarker in prostate cancer patients [18,38,57,58].
MiR-21 is upregulated and functions as onco-microRNA which is negatively modulating the expression of tumor suppressor genes; it is considered as a regulator of androgen receptor. 30 radical prostatectomy (RP) patients (14 patients with rapid biochemical failure (BF) and 16 patients without BF) with Gleason score 7 were analyzed for 1435 miRNAs. MiR-21 expression was significantly upregulated in patients with BF as compared to non-BF group (p = 0.05). The stromal expressions of MiR-21 had predictive impact on biochemical failure-free survival (BFFS) and clinical failure-free survival (CFFS). Elevation of MiR-21 expression was an independent prognostic factor for BFFS patients with Gleason score 6. For this type of patients MiR-21 may help to predict the risk of future disease progression [59-64]. In another study 56 patient serum samples (20 localized PCa, 20 androgen-dependent PCa, 10 hormone-refractory PCa administered docetaxel-based chemotherapy and 6 BPH) were tested for MiR-21 expression. It revealed that the expression of serum MiR-21 was correlated to serum PSA level in patients with ADPC and HRPC (P=0.012 and 0.049) whereas there was no significant difference in serum MiR-21 level between BPH, localized CaP and ADPC. The serum MiR-21 was elevated in HRPC patients, who were resistant to docetaxel-based chemotherapy. Thus this miRNA could be used as a marker to indicate the transformation to hormone refractory disease, and a potential predictor for the efficacy of docetaxel-based chemotherapy [51].
The miRNA profiling of serum samples from 36 African (24 PCa patients and 12 controls) and 36 caucasian American (16 PCa patients and 20 control) patients affected by PCa was studied. Three miRNAs namely MiR-25 (p< 0.01), MiR-101 (p< 0.001) and MiR-628-5p (p< 0.0001) showed low expression in serum of PCa patients as compared to normal. Among all three miRNAs, MiR-628-5p was significantly downregulated in all PCa patients. This revealed that MiR-628-5p could be developed as a non-invasive biomarker for PCa diagnosis and prognosis [65].
MiR-128 functions as tumor suppressor in neuroblastoma, glioma and PCa and is regarded as a negative regulator of malignant phenotype of PCa such as proliferation, cell motility, invasion, apoptosis, and self-renewal. MiR-128 was significantly decreased in both serum and tissue of PCa patients compared to normal samples; this was associated with aggressive clinical pathological features like advanced pathological stage, positive lymph node metastasis, high preoperative PSA and positive angiolymphatic invasion. Moreover, MiR-128 expression was an independent prognostic factor for biochemical recurrence (BCR) free survival of PCa patients. Therefore MiR-128 can be developed as a noninvasive biomarker for PCa prognosis and diagnosis [15,19,66-68].
Recent study from149 PCa patients, 57 healthy controls, and 121 BPH and other urinary diseases also showed this.
Many epidemiological studies reported the connection between herpes virus infection and prostate cancer risk as this virus inhibited cell apoptosis and stimulated DNA synthesis. Virus miRNAs are used to control the expression of either the host’s genes and/ or their own. The first virus miRNAs were found in cells infected with EBV. About 95 % of virus miRNAs known today are of herpes virus origin. In one of the recent studies of 1052 urine, 150 serum, and 150 prostate tissue samples from PCa or BPH patients overexpression of herpes virus miRNAs in urine samples from prostate cancer patients was detected compared to control subjects. This study revealed that hsv1-MiR-H18 and hsv2- MiR-H9-5p detected in urine samples had better diagnostic biomarker performance than tPSA levels in prostate cancer patients [16,69-73].
In prostate cancer diagnosis biomarker development is of urgent need. Many researchers have shown the potential role of microRNAs as biomarker in prostate cancer. A number of significant dysregulated microRNAs and aberrant expression of micro RNAs have been reported which are closely related with progression of malignancies of prostate cancer. Specific identification or detection of microRNAs in serum, plasma and urine of prostate cancer patients has been done using various techniques. These developments indicate that circulating microRNAs have potential as biomarkers in prostate cancer.
The authors are grateful to Entomology Research Institute, Loyola College, Chennai, for financial assistance. We also thank ICAR-Research complex for NEH Region, Umiam, Meghalaya and North Eastern Indira Gandhi Regional Institute of Health & Medical Sciences, Shillong, Meghalaya for support in manuscript preparation. We are indebted to Miss Thotyachan Ch from D.M. College of Science, Imphal for helping in collecting research papers.