Cold-Shock Stress Induces Formation of a Biopolymer Flocculant by Bacillus alvei NRC-14 under Static Incubation Conditions
Bacillus alvei NRC-14, a soil bacterial isolate, was found to exhibit multiple-adaptive response when exposed to abiotic stress factors. In the present study, chemical and flocculation properties of a viscous biopolymer flocculant produced by the strain under cold-shock stress are investigated. Addition of sucrose or starch at a 1% concentration increased the growth and viscosity. The biopolymer flocculant is a polysaccharide as indicated by the sugars content. Maximum values of viscosity and flocculating activity were obtained at a pH range from 5-9. Acidic or alkaline pH values reduced both of the viscosity and flocculating activity. IR-spectra revealed various active and functional groups such as carboxyl, hydroxyl, methoxyl, and urinate by which strong and large flocs are formed. The biopolymer flocculant showed thermal stability and good flocculating properties.
Keywords: Bacillus alvei; Cold Shock; Heat Shock; biofilm; Biopolymer Flocculant; Green Biotechnology
Temperature is one of the most important environmental factors for life as it directly influences structural and hence functional properties of cellular components. After a sudden exposure to a heat shock, bacteria respond by expressing a specific set of genes [1]. This heat shock response comprises the expression of protein chaperones and proteases. On other side, exposure to high temperature may lead to the formation of a biofilm as a form of self-protection. During biofilm formation, some of the cells are adsorbed to the surface for only a finite time, before being deadsorbed, in a process called “reversible adsorption” [2]. Some of these reversibly adsorbed cells permanently bind to the surface, form micro-colonies within few hours, and produce a polymer matrix around the micro-colonies in irreversible steps for a lengthy stay [3].
Rapid temperature downshifts to 10 °C can cause global alterations in gene expression that result in broad physiological changes collectively referred to as cold shock responses. These include changes in cell wall/membrane composition, protein synthesis rates, energy metabolism, and others [4]. The term ‘cold shock’ refers to the exposure of an organism to a sudden decrease in temperature, and the cellular response to this shock is called “cold shock response”. Cold shock responses have been documented in almost all unicellular organisms from thermophiles [5,6] to mesophiles [7,8].
Bacteria express a well-defined set of proteins after a rapid decrease in temperature. This protein set, however, is different from that expressed under heat shock conditions. It is expected that, the majority of the cellular processes observed after fast/long temperature shifts are also occurring during the acclimation (known as adaptation phase). A specific set of genes is induced immediately after temperature downshift and the production of defected proteins (non-cold shock proteins) in the cells becomes transiently inhibited [9]. The initial phase of cold-shock response, the acclimation phase, is characterized by high levels of newly synthesized cold-shock proteins (CSPs). At the end of acclimation phase, syntheses of CSPs decreases to a constant level and the cell restore their protein production machinery [9,10].
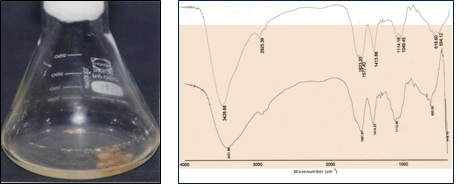
Exopolysacchrides (EPSs) are naturally macromolecules formed during growth of many microorganisms. Their composition, structure, biosynthesis, and functional properties have been extensively studied [11-14]. Some EPSs exhibit flocculating properties. Flocculants are chemicals that stimulate flocculation by aggregation of colloids and other suspended particles, forming a floc. Flocculants are widely applied in various industrial processes, including wastewater treatment, downstream processing, beverages as well as food and fermentation processes [12]. Although chemical flocculants have been widely used due to their effective flocculating activity and low cost, some synthetic flocculants are known to be hazardous to the environment. In recent years, utilization of microbial flocculants has been promoted due to their biodegradability and their environmentally inert nature [15]. The bacterial strain Bacillus alvei NRC-14 exhibits multiple-adaptive response when exposed to abiotic stress factors. This strain synthesized a biopolymer flocculant (BPF) that has chitosan-like structure (Figure 1) when grown at 30 °C [16]. The strain was also found to produce an antimicrobial agent when exposed to 40 °C under shaking culture condition [17]. During experiments by strain NRC-14, it was noticed that a biofilm is formed by the strain under static incubation after approximately 20 hrs at room temperature (39±2 °C). Interestingly, when this culture was kept at refrigerator condition (4 °C), a viscous biopolymer was synthesized, whose viscosity increased by time. Attention was turned for recovery of this biopolymer. Therefore, the present study was focused on evaluation of the different carbon and nitrogen sources for formation of this biopolymer flocculant. Purification and some properties of the produced biopolymer flocculant were also investigated.
The strain B. alvei, isolated from Egyptian soil as a potent chitosanase producer [18], was used in the present study. It was maintained on nutrient agar slants at 4 °C, with monthly transfers using chitosan-containing slants to retain viability. Prior to use, cultures of the strain was grown in nutrient broth to the log phase and repeated for 3 rounds to enrich for the determination of heat- and cold-resistance. For abiotic stress tests, the strain was grown in a medium containing: (% w/v), sucrose, 1.0; NaCl, 0.3; (NH4)2SO4, 0.2; K2HPO4, 0.3; and MgSO4.7H2O, 0.05 with adjusted pH to 6.0 [13]. Growth of the cells was monitored by spectrophotometer at 660nm. Using this medium, different modifications for growth and BPF formation were tested such as different incubation times, different pH values as well as various carbon and nitrogen sources. Carbon sources tested were glucose, sucrose, starch, and powdered chitosan. Nitrogen sources used were NaNO3, NH4Cl, NH4NO3, (NH4)2SO4, as well as yeast extract and peptone. Media were distributed in 30 ml portions into 250 ml Erlenmeyer flasks and inoculated with 1 ml of a 24 hrs culture grown in nutrient broth medium (O.D660=2.5). In all experiments, the cultures were pre-incubated at room temperature (39 °C ±2) for 24 hrs without shaking, followed by keeping at refrigerator condition (4 °C).
For recovery and purification of the BPF, cultures (400 ml) were centrifuged at 8000 x g and 4 °C for 30 min. The BPF was precipitated from the supernatant by addition of five volumes of cold ethanol and allowed to stand overnight at refrigerator conditions. The solution was centrifuged at 8000 x g and 4 °C for 30 min and the resultant precipitate was collected, dialyzed against distilled water, and lyophilized. The purified viscous material was kept at 4 °C and used for analytical and flocculating properties studies [19].
Protein content was assayed by the method of Lowry, using bovine serum albumin as a standard [20]. The content of amino-sugars in the BPF was measured by the Elson-Morgan method [21] using glucosamine as a standard. Sugars content was determined by the method of Dubois et al. [22]. Viscosity of the BPF solution was determined using a Brookfield viscometer type LV (at 50 rpm).
The flocculating activity (FA) of the BPF was measured according to the method reported by Kurane et al. [23] using a suspension of kaolin (5g/L, in distilled water) as a test material. Briefly, 2 ml of biopolymer flocculant solution were added to 100 ml kaolin suspended solution. The mixture was vigorously stirred and poured into a cylinder and allowed to stand for 10 min. The optical density of the clarifying solution (A) was measured with a spectrophotometer at 550 nm [15]. A control experiment was prepared using the same method, except that the bioflocculant solution was replaced with distilled water (B). The flocculating activity was measured using the equation:
Flocculating activity (%) = [(B – A)/B] × 100
Where A is the absorbance of the sample experiment at 550 nm, and B is the absorbance of the control at 550 nm.
The produced BPF was analyzed by infrared using a FT-IR-FT Raman (Nexus 670, Nicolet-Madison-WI-USA). The spectrum of the sample was recorded on the spectrophotometer over a wave number range 4000-400 cm-1. The IR-spectra of the produced BPF was detected by the “Central Services Laboratory”, National Research Center.
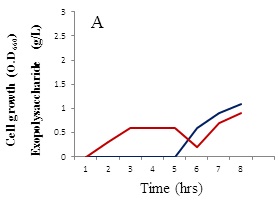
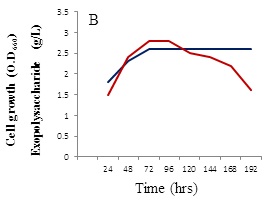
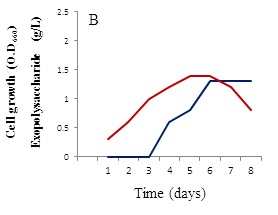
The growth behavior of B. alvei NRC-14 under heat and cold shock stress was studied. When exposed to high temperature and cold stresses under static incubation, the strain adapted to these changes (Figure 2). When kept at room temperature (39±2 °C), it formed a biofilm as a form of self-protection (Figure 2A), after which a “reversible adsorption” took place, where some bacterial cells removed and adsorbed to the surface of the bottle to reproduce and form micro-colonies, in an irreversible steps (Figure 2B). This process was followed by exposure of cells to a cold shock where the bottles were kept at refrigerator condition (4 °C). Surprisingly, a highly viscous biopolymer was synthesized by the strain (Figure 2C). On other hand, after exposure to the cold-shock, cell growth at exponential phase stops for several hours to adapt for acclimation period (3-5 hrs), after which cell growth dropped for a short period (Figure 3A). Production of the BPF was detected after approximately 20 hrs, rising as cell growth increased and reaching a maximum (2.6 g/L) after 96 hrs (Figure 3B).
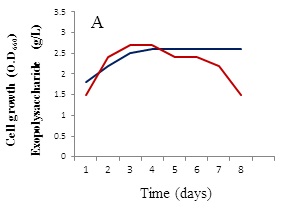
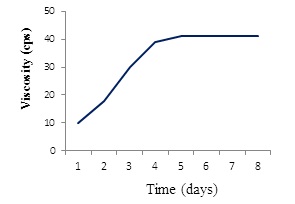
Effect of different carbon and nitrogen sources were tested for cell growth and formation of the BPF. Sucrose was the best carbon source (Figure 4A) in comparison with powdered chitosan, where growth of the strain was obviously decreased (Figure 4B). Results revealed also that (NH4)2SO4 was the best nitrogen source for cell growth and BPF formation (data not shown). On other hand, viscosity of the BPF increased over time (Figure 5). Viscosity values obviously increased during the incubation time and maximum value (39 centipoises) reached after 96 hrs (Figure 5), after which these values were quite similar up to 8 days.
The main backbone of the purified BPF produced by strain NRC-14 is a polysaccharide as indicated by the carbohydrate content. In this respect, complete hydrolysis of the viscous BPF was carried out with 2N HCl at 100 °C. Results showed that, the majority of constituents in the HCl hydrolysate were aminosugars (73.6 %) besides a low amount of protein (1.2 %).
Flocculating properties test of the purified BPF showed good flocculating activity; efficacy for aggregation and precipitation of kaolin particles reached 99.3% after 10 minutes of keeping at room temperature (Figure 6).
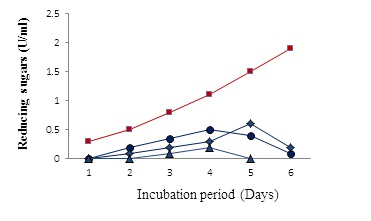
It was of interest to study the stability of the purified BPF against degradation by enzymes. In the present study, a variety of purified enzymes (chitinase, chitosanase, β-1,4 –glucosidase, and protease produced in our lab) were tested for degradation of the BPF, individually, or in combination. Results revealed that: firstly, individual use of chitosanase, β-1,4 –glucosidase, or protease resulted in degradation of the biopolymer to some extent as indicated by detection of reducing sugars (Figure 7). Secondly, an obvious degradation of the biopolymer was achieved when a mixture of these enzymes was used. Pronounced amounts of reducing sugars are, however, detected after a long period (Figure 7).
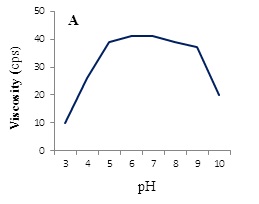
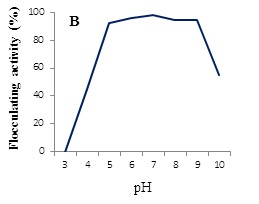
Effect of pH on viscosity and flocculating activity of the BPF was tested. Maximum values of both viscosity of the solution (10% v/v, in distilled water) and flocculating activity are obtained at pH values ranged from 5-9, while they were decreased at either acidic or alkaline conditions (Figure 8A and B).
Temperature dependence of the BPF revealed that its FA could effectively occur at temperatures ranging from 30-90 °C. Below 20 °C, the purified BPF acquired a mucoid and ropy property; therefore, recovery steps of this BPF were performed with cold distilled water and ethanol. On other hand, the heat-stability of the BPF revealed that it is a heat-stable polysaccharide, which remained about 97.8% of its FA after heating at 100 °C for 20 min (data not shown).
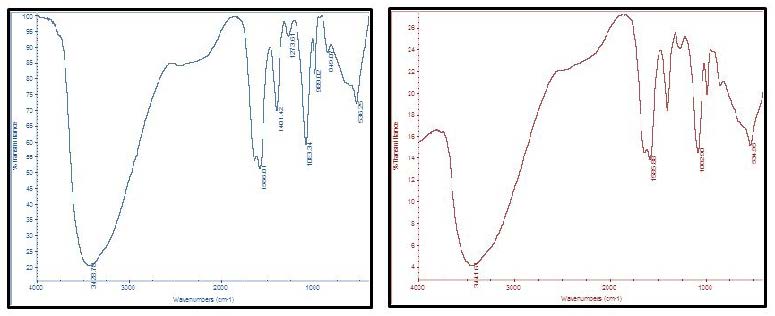
The infrared spectra of the purified BPF displayed a stretching band at 3441 cm-1 characteristic of hydroxyl and amino groups (Figure 9). An asymmetrical stretching band observed at 1585 cm-1 is characteristic of C=O stretching vibration in –NHCOCH3. The band observed at 1401 cm-1 indicated the presence of urinate. The adsorption peak at 1273 cm-1 indicated the C-O stretching vibration and presence of methoxyl groups. In addition, the band at 1083 cm-1 is generally known to be typical characteristics of all sugar derivatives. The small absorption bands at 849 cm-1 and 989 cm-1 could be associated with β -glycosidic linkages between the sugar monomers.
All microorganisms must possess the ability to adapt to environmental changes that include changes in nutrient availability and general parameters such as osmotic pressure, high or low temperature, acid or alkaline pH values. Growth of the strain NRC-14 during cold shock and formation of the biopolymer flocculant was examined. When exposed to a cold-shock stress, cell growth after the exponential phase was stopped for a few hours (acclimation period) and then decreased and re-enhanced. After acclimation period for synthesis of CSPs, growth increased and reached a maximum after 96 hrs (Figure 3).
Several studies with bacteria indicated that some cold-shock proteins appear very rapidly following temperature downshift and increase during post-shift to reach maximal induction before or at mid-lag phase. A similar situation existed in E. coli in which CSPs were very quickly induced after a temperature downshift from 37 to 10 °C, with an optimal level of induction towards 2 hours [24]. Different mechanisms exist for bacteria to respond to temperature downshifts, and the time to re-adapt to the low temperature is not directly dependent on the range of the bacterium growth, i.e., of its psychrophilic, psychrotrophic, or mesophilic character [25]. In this respect, the strain NRC-14 is interesting since, while being able to grow at temperatures close to or below freezing, it kept its ability to withstand mild and high temperatures. Moreover, the strain frequently submitted to large and rapid temperature changes and can develop over a wide temperature range (more than 40 °C). This ability to cope with such temperature shifts must be accompanied by adaptive changes in response to alterations of numerous physical and biochemical parameters, including solubility, reaction kinetics, membrane fluidity, protein conformation and stability and changes in gene expression [25,26]. Therefore, genotypic adaptation to very low temperatures may represent the sum of many end-points of phenotypic adaptations of cell structure/metabolism, interacting and biochemical effects, which can be achieved to various extents by different microorganisms. Investigation of the adaptation process of microorganisms can provide important information with applications in industry and in health. Horn et al. [9] reported that, during acclimation phase, bulk protein synthesis stops (non-cold-shock proteins), while the small fraction of cold-shock proteins (CSPs) important for adaptation is expressed (synthesis of CSPs). After the acclimation phase, growth and normal protein production resumes although at a slower rate, and the biosynthesis of most of the CSPs is stopped.
Cell growth and formation of the BPF by the strain NRC-14 were affected by the carbon source. Sucrose and starch induces formation of the BPF rather than powdered chitosan. The strain under abiotic-stress conditions consumed the more-easier carbon source (sucrose or starch) rather than the most complex (chitosan) to quickly adapt for hard condition as a form of self-protection. So, both of the growth and biopolymer flocculant formation were high with the simple medium (Figure 4A and B).
Stirring/aeration conditions influenced formation of a BPF by bacteria. Aeration of culture medium is an important requirement for most polysaccharide-synthesizing bacteria, whether they are aerobes or facultative anaerobes [27]. However, in the present study, the polysaccharide yield, viscosity, and flocculating properties were higher than that obtained in our previous work under shaking conditions [16]. This result is wondrous and amazing, as shaking and stirring are favored for bacterial growth. Moreover, strain NRC-14 can adapt with static conditions and grow in low nutrition medium, which indicates that it is not an exigent strain. The backbone of the produced biopolymer was sugars rather than proteinaceous molecules as indicated by determination of sugar content.
The biopolymer produced by strain NRC-14 is highly stable and may contribute for the cell survival under abiotic stress conditions. It requires a complex of enzyme preparation which could act synergistically to degrade several bonds in the carbon chain. It is well known that most enzymes that capable of degrading polysaccharides are belonging to the glycosidase family (amylase, cellulase, chitinase, chitosanase, etc). So, unlike most microbial polysaccharides, the BPF produced by strain NRC-14 is a biologically highly stable polymer. The protection of cells may, probably, be due to the stability of this BPF. The result of this study is in accordance with previous observations reported by Pirog et al. [28] for an EPS from Acinetobacter sp. They explained that an enzyme alone may only be specific for a certain chemical bond in the carbon chain of an EPS, and that a complete degradation of an EPS, probably, requires a complex of enzyme preparation or series of enzymes produced by mixed cultures.
Some EPSs exhibit flocculating properties. Components and structures of bioflocculants are complex, and different flocculants produced by microorganisms can have different properties. A large molecular-weight bioflocculant is usually long enough and has sufficient number of free functional groups by which strong and large flocs are formed [29]. Free functional groups can act as bridges to bring many suspended particles together. The mechanism of flocculation in biological system is highly complex and depends on many interacting variables such as temperature, pH, microbial species and medium components [30,31]. Effect of pH on viscosity of the BPF showed maximum values at pH ranged from 5-9, while at acidic or alkaline pH values a pronounced decrease was occurred. This result may be due to the change of molecular conformation of the polysaccharide chains affected by a net electric charge of the polysaccharide molecule [32].
IR-spectrum was used to compare the original BPF samples with those obtained after thermal treatment. The spectra presented in Figure 9 showed no obvious differences between the two samples. The small variations that occurred in the spectra were not significant and were probably associated with the mild mass loss, i.e., removal of hydration water [33]. IR-spectra of the BPF produced by strain NRC-14 showed presence of carboxyl, hydroxyl, methoxyl and amino groups which are the preferred groups for the flocculating process, similar to those observed in results reported previously [30,34-37]. The carbohydrate content of this biopolymer may explain its stability towards organic solvents (acetone, methanol, and ethanol). The insolubility of polysaccharides in organic solvents may be due to the increased number of carboxyl groups, hydroxyl groups, and urinate which enhances the formation of hydrogen bonds and building up strong forces of attraction between the polysaccharide molecules resulting in the crystalline polymer solids [35]. These forces are hard to be broken by organic solvents or enzymes, which might be responsible for thermal stability of the BPF [15,35,38]. These groups, together with the amino group, could serve as a binding site and are likely to be the preferred groups for the process of adsorption [39].
The term “biopolymers” usually describes polymers produced in a natural metabolic way by living microorganisms. The molecular backbones of these biopolymers are composed of repeating units of polysaccharides, nucleic acids, or amino acids which are quite suitable for high-value medical applications such as tissue engineering and drug delivery [40-42]. Nowadays, green biotechnology, green products, and green chemicals have gain more and more importance in the industrial practice. Large-scale production of biopolymers has been an emerging branch of industry for decades [43]. The development of such green biotechnology has transformed microbes into useful factories for producing valuable polymers from renewable biomass. Recent progress at the interface of chemistry and biology has enabled the production of a variety of new biopolymers with properties that substantially differ from synthetic polymers [44]. Some of the more biopolymers such as γ-polyglutamic acid, bacterial cellulose and xanthan gum are mainly produced by microorganisms [42].
Enzyme- and metabolic-engineering approaches towards the production of biopolymers are reported [45]. Metabolic engineering has become more powerful, through the integration of itself with systems and synthetic biology. Systems biology is a relatively new approach to biological research which looks at the complex interactions within whole cell systems. It allows cell-wide understanding of metabolic reactions and the way these are regulated by the cell’s genes [46]. Synthetic biology is another new approach that designs and constructs new biological functions and systems that aren’t found in nature. It allows the design of new genes, modules and circuits that can be used to modulate the cells metabolism to make more of the desired bio-products. So systems metabolic engineering can now develop superior microorganisms much more efficiently through the integration of itself with systems biology and synthetic biology [46].
A more challenging trend in biopolymer production is the switch from traditional extraction or conversion of natural products to recombinant/heterologous production techniques in microorganisms. Despite the complexity of these biopolymers in structure and production, all share important features such as biocompatibility, adjustable shapes and slow biodegradation. The combination of properties renders these biopolymers ideal materials for biomedical scaffolding, surgery, sutures, and wound healing as well as related pharmaceutical applications, drug delivery, and tissue engineering [40,43,47]. The fields of tissue engineering and regenerative medicine are growing exponentially through better technology and research. Biopolymers such as cellulose, chitosan, and chitin are used in drug delivery, tissue engineering, wound care as well as other pharmaceutical fields [47]. Recently, bacterial cellulose has significant potential as a food ingredient in view of its high purity, in situ change of flavor and color, and having the ability to form various shapes and textures. Its material properties are multifunctional, with potential uses for thickening and gelling, stabilizing, water-binding, and as a packing material [44,48].
The strain B. alvei NRC-14 has proved to adapt with various stress conditions. This bacterial strain has adopted special metabolic pathways to survive in extreme conditions and so has better capacity to produce special bioactive compounds. In fact, this strain is particularly interesting because of: 1) the large quantities and variety of extracellular metabolites it produces; 2) controlling its growth conditions using minimum nutrient substrates; 3) synthesizes biofilm as a form of self-protection when exposed to high or low temperatures. In the present study, sucrose or starch induced formation of the biopolymer flocculant which is found to be stable against degradation by some enzymes and characterized by thermal stability. In addition, the purified biopolymer solution was highly viscous. Generally, biopolymer flocculant from the novel strain B. alvei NRC-14 are heat-stable, highly viscous, and possesses a good flocculating property. Such biopolymer flocculant can be optimally produced and applied in the fields of biotechnology.