Comparison of In Vivo and In Vitro Tests of Resistance in Plasmodium Falciparum Positive Patients Treated With Artemisinin Based Combination Therapy in Northwestern Nigeria
Comparative studies on in vitro and in vivo drug response of Plasmodium falciparum isolates were carried out among malaria positive resident of Kano and Katsina states of Nigeria, to determine the effectiveness of in vitro antimalarial drug sensitivity test as a substitute for in vivo therapeutic response analysis. The in vitro test was carried out using Schizont growth inhibition assay which was evaluated by comparing its results with the in vivo/therapeutic response determined by 28 days follow-up of the Plasmodium falciparum positive patient treated with different Artemisinin combination therapy (Artemether-lumefantrine, Dihydroartemisinin-piperaquine, Artesunate-amodiaquine). Out of 652 patients enrolled, 227 (34.8%) completed the 28 days follow-up, and 120 isolates from subjects with complete follow-up data yielded an interpretable in vitro test. A total of 100 of 120 patients (83.3%) had adequate clinical and parasitological response. The geometric mean 50% inhibitory concentrations (IC50) of the isolates obtained from these patients were 2.03nM, 3.65nM and 4.68nM for Artemether-lumefantrine (AL), Dihydroartemisinin-piperaquine (DHP) and Artesunate-amodiaquine (AA) respectively (in vitro and in vivo sensitive). Treatment failure was observed in 20 (16.7%) of 120 patients whose IC50 values were 2.11nM, 3.77nM and 4.80nM for AL, DHP and AA respectively. Moreover all the isolates of the patients responding with treatment failure yielded a discordant result (i.e. in vivo resistance and in vitro sensitive). Thus, the result of this study indicates poor agreement between the in vitro and in vivo test (Kappa value = 0) with regards to treatment failure. The in vitro assay cannot therefore be used as a substitute for in vivo therapeutic test for drug efficacy.
Keywords: Acts; Malaria; In Vitro; In Vivo; Plasmodium Falciparum
Malaria is a mosquito-borne infectious disease caused by an intracellular protozoan parasite of the genus plasmodium [25]. Five species of plasmodia namely; plasmodium falciparum, P. vivax, P. ovale, P. malanae and P. Knowlesi, cause the disease in humans. The most serious forms of the disease are caused by Plasmodium falciparum [1]. Resistance of P. falciparum to the traditional antimalarial drugs (Chloroquine and Sulpadoxyne-pyrimethamine) is a growing problem and is thought to have contributed to increased malaria mortality in recent years [2]. In Nigeria as well as other countries, malaria treatment and control are hindered by the spread of resistance to common antimalarial drugs. This increasing drug resistance has necessitated change in antimalarial therapy in Africa. This has led the World Health Organization to recommend the use of Artemisinin combination therapy (ACT), which is highly efficacious.Multiple highly effective ACT regimens are now available, but the optimal choice for malaria in most areas remains uncertain [3]. ACT has been promoted to be an effective strategy to combat the emergence and spread of resistance. However evidence of resistance has already emerged in some, parts of the world [3]. Decreased sensitivity to ACTs is alarming since there is no alternative class of antimalarial ready to replace the Artemisinin derivatives. Thus the world health organization (WHO) has launched an intensive campaign to monitor ACT resistance [3].
Four different ways of monitoring and reporting parasite susceptibility or resistance to antimalarial drugs exist. These include the in vivo tests, the in vitro test, the use of animal models and molecular characterization [4]. In vivo and in vitro test of resistance are the most commonly employed methods in the field [5].
Generally the in vivo test has been the standard test normally used in making policy decision, concerning the efficacy of a given drug in an area or region. However, the in vivo tests are time consuming and require that subjects participating in the test should be under periodic treatment and examination for a minimum follow up period of between 14-28 days. The test is also affected by factors such as the patient’s immunity, variation of drug absorption and metabolism, and possible misclassification of new infections as recrudescence [6]. These necessitate the need to determine the effectiveness of in vitro antimalarial drug sensitivity tests as a substitute for in vivo test.
This study was aimed at comparing the in vivo and in vitro test of resistance in plasmodium falciparum positive patients treated with different ACTs, with a view to evaluate the possible use of in vitro antimalarial drug sensitivity tests as a substitute for therapeutic response analysis.
The study was conducted at some Hospitals in Kano and Katsina, Nigeria in 2014. Malaria is hyper endemic in the study area with high transmission intensity during rainy season (April to October).
The study protocol was approved by the ethical committee of Kano and Katsina state hospital management board. Informed consent was obtained from parents or guardian of the study children while assent was obtained from the adults.
Patients of all ages with symptoms of uncomplicated malaria including a fever or history of fever within 48 hours and mono infected with P. falciparum of ≥ 2000 asexual parasite /μl of blood participated in this study. Subject with symptoms of severe malaria, a recent history of use of antimalarial drugs, presence of other diseases and reported allergies to the study drugs were excluded from the study.
The study drugs include Artesunate-amodiaquine (Novartis),Dihydroartemisinin-piperaquine (WAIPA, Novartis,) and Arthemeter-lumefantrine (Novartis).
Subjects enrollment, blood sample collection, treatment, follow- up and other laboratory procedures were carried out according to the procedure of [3,7,8,26]. Recruited subjects with prescription of any of the above ACTs were treated and followed up for 28 days. A part from first dose drug administration was not supervised. The drugs were administered orally according to body weight for three days: Arthemeter-lumefantrine (20mg:120mg) administered as one tablet to subjects of 5-14kg, two tablets to 15-24kg, three tablets to 25-34kg and four tablets to subjects > 35kg given twice daily. Dihydroartemisinin-piperaquine (40mg dihydroartemisinin/320mg piperaquine) tablets were given as half to one tablet to subject 5-14kg, two tablets to 15-24kg, three tablets to 25-34kg and four tablets to subjects > 35kg once daily. Artesunate-amodiaquine (100mg/270mg) was also administered according to body weight as half tablet to 5kg to 8.9kg, one (9-17.9kg), 1½ (18-34kg) and 2 tablets to subjects >35kg daily.
Recruited subjects were asked to return to the health centers for clinical and parasitological response evaluation on day 3, 7, 14, 21 and 28 post treatments. They were also advised to return at any other day if the sickness persisted. Some of the patients who did not turn up for scheduled follow-ups were visited at home. Patients were excluded during follow up for use of another antimalarial drug, serious adverse events requiring a change in treatment and withdrawal of informed consent or loss of follow up. Blood samples were taken on each follow-up day via finger prick to identify parasite clearance through microscopic examination of thick and thin Giemsa stained blood films. Treatment responses were recorded as classified by [10], early treatment failure ETF (parasitaemia > 25% of day 0 count and temperature >37.5ºC), late clinical failure LCF (Present of parasitaemia after day 3 with axillary temperature >37.5ºC), Late parasitological failure LPF (Present of parasitaemia after day 3 with axillary temperature <37.5ºC) and adequate clinical and parasitological response ACPR (absence of parasitaemia on day 14 irrespective of axillary temperature).
Blood samples obtained before treatment were tested for in vitro sensitivity to different artemisinins combination drugs mentioned above using Schizont growth inhibition assay described by [9,10].
Different concentrations of ACTs in the appropriate solvent were distributed in 96 wells micro culture plates and dried in an incubator. The in vitro cultivation of the parasite was achieved using RPMI 1640 medium following modification of the standard culture techniques [11]. Briefly the culture media is made up of 10.43g RPMI 1640 (sigma), supplemented with 5% albumax II (Gibco) and buffered with 5.96g Hepes and 25mM NaHCO3 (sigma Aldrich) per liter of double distilled water. The medium was sterilized by filtration through 0.22μm filter and 0.5ml of 50mg/ml gentamicin was added to inhibit bacterial growth. Malaria infected blood from each subject was suspended in 1640 medium in 1:20 dilution. Two hundred microliter (200μl) of the mixture was transferred to wells of micro culture plates predosed with varying concentration of antimalarial drugs (ACTs), including drug free control wells. The plates were incubated for 30 hours in a candle jar at 37 oC under reduced oxygen. Thick blood films were prepared from each well after incubation and stained with 2.5% Giemsa stain according to the procedure of [26]. The number of schizonts formed in each well per 2000 a sexual parasite was counted and recorded. TheIC50 of ACTs against the isolates was determined from dose response curves according to the procedure of [12]. Mean % of the parasite inhibition in vitro was calculated as 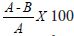
The results of in vitro assay were expressed as 50% inhibitory concentration (1C50). In vitro resistance threshold was determined according to [11] as > 2 X standard deviation above the mean.
Statistical analysis was performed using SAS software general linear model version 9.3, OpenEpi version 2.3, and nonlinear regression software (HN-Nonlin). The level of significance (p) was fixed at 0.05; parameters were compared between patients using T-test, ANOVA and Chi-Square. The results of in vivo and in vitro tests of resistance was compared using kappa statistics to calculate the index of agreement interpreted as 0-0.2, slight agreement; 0.21-0.40, fair ; 0.41- 0.60 moderate; 0.61-0.80, good and > 0.81 very good agreement [5].
A total of 1536 subjects presented to the study hospitals with symptoms of malaria were screened. Six hundred and fifty two subjects met the study criteria and were enrolled in the 28 days follow-up. Table 1 represents the summary of the enrollment and the outcomes.
Among the 652 subjects enrolled, 227 (34.8%) completed 28 days follow up and 425 (65.2%) lost to follow-up. Two hundred and one (88.5%) of the subjects who completed 28 – days follow-up had adequate clinical and parasitological response (ACPR) and 26 (11.5%) failed the treatment.
Therapeutic characteristics/clinical parameters of the subjects who completed 28 days clinical study were compared between patients with an adequate clinical and parasitological response and patients with treatment failure presented in Table 2. The mean age, duration of symptoms before treatment and parasitaemia values of subject with ACPR (10.8 years, 4.4 days, 15,600 parasites/μl) were found to be significantly different (P<0.05) from that of subject with treatment failure (4.54 years, 6.9 days and 19,980 parasites/μl) respectively.
The In vitro drug susceptibility tests were performed on 652 isolates of Plasmodium falciparum, 436 (67%) from Kano and 216 (33%) from Katsina. One hundred and fifty (23%) of the cultured isolates grew in vitro and yielded complete results for the in vitro drug susceptibility tests (Table 3). The proportions of successful assay were 21% (n=90) and 28% (n=60) for Kano and Katsina respectively.
The percentage growth inhibition of each antimalarial was dose dependent and increased with increasing drug concentrations (Table 4). The three (3) ACTs (Artemther-lumefantrine, Dihydroartemisinin-piperaquine, and Artesunate-amodiaquine) each achieved 100% parasite growth inhibition at higher concentrations. However, there was no significant difference in the level of parasite growth inhibition in vitro among the three drugs, at the various concentrations used (X2 = 15.63, df = 12 P>0.05).
Artemether-lumefantrinehas the lowest IC50 value of 2.04nM, followed by DHP with IC50 value of 3.67nM and AA have the highest IC50 value of 4.70nM. The geometric mean IC50 value of all the ACTs against P. falciparum isolates of subjects from Kano and Katsina were not statistically different P>0.05.
The In vitro geometric mean IC50 values for isolates obtained from 100 patients with an adequate clinical response was 2.04nM for AL, 3.67nM for DHP and 4.70nM for AA (Table 5). Among the isolates obtained from patients with treatment failure (n=20), the geometric mean IC50 values were 2.11, 3.77 and 4.80nM for AL, DHP and AA respectively. Thirty (30) isolates were from subject with unknown in vivo therapeutic status and their IC50 values were found to be 2.09nM (AL), 3.79nM (DHP) and 4.65nM (AA) respectively. There was no statistical difference in the IC50 values of all the ACTs against isolates from subject with ACPR, treatment failure and subject with unknown therapeutic response P>0.005. The IC50 values of all the ACTs tested against the P. falciparum isolates are below the resistant threshold.
The in vitro susceptibility patterns of the three ACTs against P. falciparum parasite isolates at both Kano and Katsina demonstrated that all the isolates were 100% sensitive (Table 6).
The validity of the in vitro results determined using the threshold IC50 value for ACTs resistance (2.88nM for AL, 4.51nM for DHP and 5.56nM for AA respectively), as compared with the therapeutic response is shown in (Table 7). The In vitro and in vivo results were compared using the Kappa statistics. The overall Kappa statistics (Kappa coefficient) for agreement between In vivo and In vitro test was 0.0 in the present study, thus demonstrating poor /slight agreement between the two methods.
Monitoring ACT treatment response for early detection of resistance is an important issue in malaria control. The in vitro and in vivo activities of different ACTs were evaluated and compared in this study. The in vivo aspect of this study revealed high ACT treatment failure (11.5%) compared to 7% failure rate reported in western Nigeria [13] and 5.2% in Tanzania [14] in a 42 days follow- up study. The failure rate (11.5%) observed in this study is similar to the failure rate observed in Tanzania of 11.2% during 28 days follow-up by [15]. The higher failure rate observed in this study could be explained in a number of ways. Firstly, molecular genotyping of pretreatment and post treatment parasite was not conducted to distinguish between treatment failure and new infections. Secondly, drug administration was not properly monitored apart from first dosage because the subjects were out patients and also blood drug levels were not determined in these patients in order to confirm these findings.
Of the total 652 parasite isolates of Plasmodium falciparum subjected to in vitro sensitivity tests, successful responses were seen in only 150 isolates, this could be due to the fact that in vitro culture of P. falciparum remains a very delicate test whose success depends on several parameters which are difficult to control, including insufficient maturation of schizonts and contamination.
All isolates tested were 100% sensitive to both ACTs (Arthemether-lumefantrine, Dihydroartemisinin- piperaquine and Artesunate-amodiaquine); which makes these regimens of choice not only in the management of cases of malaria but also in the treatment of remedies for common antimalarial drug resistance. These results are consistent with the previous report that found prevalence of in vitro sensitivity of P. falciparum to Arthemether-lumefantrine (Coartem) of up to 96.29% in Cotonou, Benin Republic [16].
The definition of in vitro parasite resistance to antimalarial drugs was based on the threshold cut-off values of IC50 of various antimalarial drugs, which distinguish resistant from sensitive parasites. Therefore the threshold IC50 values were calculated from the formula (IC50mean+2SD) [17] as 2.88, 4.51 and 5.56nM for AL, DHP and AA respectively. However, in all the isolates tested the IC50 values were found to be below the respective cut- off values for resistance.
This study reports the geometric mean IC50 values for Artemether-lumefantrine as 2.04nM, Dihydroartemisinin-piperaquine3.67nM and Artesunate-amodiaquine 4.70nM. These results are consistent with other studies from Africa reporting IC50values of Artemisinin derivatives as 2.2 and 2.6nM from Senegal, and Rwanda respectively [17,18]. In contrast to the finding of this research, some surveys around the world provide evidence of reduced in vitro susceptibility of Plasmodium falciparum to Artemisinin derivatives (Art emetherand Dihydroartemisinin) [16,20].
The result of in vitro studies, which shows 100% sensitive isolates contradicts the in vivo report of this study, this suggest poor agreement of the two methods of assessing effectiveness of ACT in northwestern Nigeria. Plasmodium falciparum isolates from subjects with adequate clinical and parasitological response (in vivo sensitive) were found to be 100% in vitro sensitive to all ACTs. The discordant results were only observed in subject with treatment failure in which all the isolates of the subjects were in vitro sensitive. This could be due to limitation of this study that fails to establish the relationship between treatment failure and in vivo drug resistance due to some factors. The factors may include patient immunity, variation in pharmacodynamics and pharmacokinetics, host genetic factors, late emergence of secondary or tertiary broods of parasite from liver and possibly misclassification of new infection as recrudescence. These factors can account for the reason of discordant results. Both in vitro and in vivo tests of resistance have their limitation and in any case do not measure the same biological phenomenon. The in vitro assays exclude several host factors that influence the results of the in vivo test. However, in view of some discordant results, the in vitro test cannot substitute for in vivo data on therapeutic efficacy [5].
According to the [21] the uses of in vitro tests provide information on the qualitative response of P. falciparum irrespective of the patients’ immune status unlike in vivo tests. Thus the in vitro tests serve as one of the useful epidemiological tools for assessing the baseline sensitivity of drugs, and for monitoring drug response of P. falciparum over time and place. The test provides the necessary background information for the development and evolution of drug policies in a given malaria control program. This is because; noticeable changes in the parasites drug sensitivity profile in vitro in follow up studies could be an indicator of a future therapeutic failure [22]. Unlike the in vivo tests, the results of the in vitro test are not equally affected by on-going malaria transmission. The tests could be conducted simultaneously with many drugs, and independent of the patient clinical condition. In vitro tests also permit the monitoring of drug response with compounds for which protocols for in vivo tests, has not yet being developed [23-25].
The IC50values of all the ACTs tested (Artemether – lumefantrine, Dihydroartemisinin-piperaquine, Artesunate-amodiaquine) against P. falciparum isolates are below the resistance threshold (2.88nM, 4.51nM and 5.56nM for AL, DHP and AA respectively). This revealed that all the ACTs are still efficacious in the treatment of uncomplicated malaria in Northwestern Nigeria. All the isolates of the patients responding with treatment failure yielded a discordant result (i.e. in vivo resistance and in vitro sensitive). Thus, the result of this study indicates poor agreement between the in vitro and in vivo test of resistance (Kappa value=0) with regards to treatment failure. The in vitro assay cannot therefore be used as a substitute for in vivo therapeutic test for drug efficacy.
