Compromised T cell Immunity in Elderly via Naïve T cell Anergy
Immunosenescence is the term commonly used to describe age-related changes in immune responses with aging. It renders older adults more prone to infectious diseases and age-related diseases like cancer, Alzheimer’s, osteoporosis, and autoimmunity. This commentary describes molecular signals that promote imbalance in the ratio of naïve and memory T cells and key findings regarding age-related defects in the function of T cells, and how the functional defect can be compensated in vaccine design.
Keywords: Immunosenescence; Memory T inflation; Aging; Immune Responses, T Cell Immunity
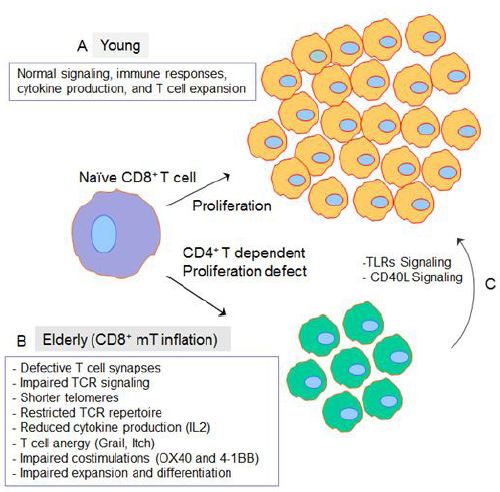
The immune system is profoundly affected by aging resulting in a condition called immunosenescence in elderly individuals (>65 years old). Immunosenescence results in increased susceptibility to infections, poor response to treatments and vaccination, increased prevalence of cancer, and also autoimmunity leading to diseases such as atherosclerosis and diabetes mellitus [1,2]. Antibody responses generated early in life, before the onset of immunosenescence, persist well into later life [3]. Similarly, T-cell memory generated during youth functions well into old age, whereas immune memory produced later in life (i.e., elderly) functions poorly [4]. Due to the accumulation of age-related defects, T-cell functions in the elderly decrease thus limiting effective immune responses against pathogens [3,5]. The general opinion is that naïve T cells become progressively dysfunctional with aging, but the memory T cells remain functionally intact [6-8]. Studies have suggested that naïve T cell dysfunction in elderly occurs due to the defects in the formation of T cell immunological synapses and early T cell receptor (TCR) signaling events [9]. Furthermore, naïve T cells from aged individuals exhibit numerous functional defects, including shorter telomeres, a restricted TCR repertoire, reduced cytokine production, and impaired expansion and differentiation into effector cells following antigen stimulation [10,11].
Two major age-related changes have been noticed in cell-mediated immunity: the reduction in thymic output (i.e., reduction in naïve T cells) and increase in the number of antigens experienced memory cells, particularly senescent cells [11]. It was reported that thymic involution, which is characterized by progressive thymic atrophy and reduced thymopoiesis, results into a decreased output of regulatory T (Tregs) cells as well, and thus might be a contributing factor to old-age related autoimmunity [11,12]. The upregulation of thymosupressive cytokines (i.e., interleukin-6 (IL-6), leukemia inhibitory factor (LIF); and oncostatin M (OSM) in aged humans and mice have been linked to thymic atrophy [13]. Reduction of IL-7 production by a stromal cell is also found to be one of the causes of thymic atrophy [14,15] because IL-7 is necessary for thymopoiesis that promotes cell survival by maintaining the anti-apoptotic protein Bcl-2 and inducing VDJ recombination [16]. Due to decreased thymic output, the number of circulating naïve T cells (i.e. CD45R+ CD28+ CD62+) decrease in the bloodstream and lymph nodes [13,17]. The decrease in thymopoiesis with advancing age causes a shift in the ratio of naïve to memory T cells [18]. Studies have demonstrated that repetitively antigen exposure (chronic infection) in elders is a major factor that is involved in the generation and maintenance of memory T cell inflation [19,20]. Firstly, it was found inflationary memory T cells show phenotypical signs of activation such as low expression of CD62L, CD127, CD27, and CD28 [2122]. In another set of experiments, authors found that the adoptive transfer of inflationary T cells into naïve recipients results in a failure to divide and survive, whereas the same cells are maintained when transferred into chronically infected mice [23]. Also, when an epitope that, in the context of murine cytomegalovirus (MCMV) or herpes simplex virus-1 (HSV-1) infection, induces T cell inflation is expressed in a recombinant vaccinia virus, memory inflation is not elicited [21]. Furthermore, it was demonstrated that once an infection is established, systemic viral production is not a prerequisite and merely the presence of the virus in a latent state is sufficient to drive memory T cell inflation [24]. Molecular signals as induced by cytokine (IL-2) and co-stimulatory molecules such as 4-1BB and OX40 also contribute to the development of inflationary MCMV- specific T cell responses because interference with such triggering has a major impact on inflation [25-28].
CD4+ T cells are vital for the development of memory CD8+ T (CD8+ mT) cell responses and even more so for inflationary responses [29,30]. In cellular immunity, CD8+ T-cell responses include CD4+ T-cell-independent and CD4+ T-cell-dependent ones [31]. In case of CD4+ T-cell help dependent response, CD40L signaling derived from CD4+ T-helper (Th) cells need to license antigen-presenting cells before generating measurable CD8+ T-cell responses to noninflammatory antigens. While in case of CD4+ T-cell help independent response, antigen-presenting cells mature via triggering Toll-like receptors (TLRs) by pathogen-associated molecular patterns of inflammatory antigens, leading to direct stimulation of CD8+ T-cell responses. Vezys, et al. investigate whether CD8+ mT-cell inflation affects late CD4+ T-cell-independent CD8+ T-cell responses using a heterologous prime-boost virus-based vaccination regimen eliciting functional mT-cell inflation [32]. Authors found that CD8+ mT-cell inflation derived from the late vesicular stomatitis virus (VSV) challenge did not affect functional CD8+ cytotoxic T cells (CTLs) primarily stimulated by lymphocytic choriomeningitis virus (LCMV) [32]. Similarly, LCMV-induced CD8+ mT-cell inflation had no impact on the late CD4+ T cell-independent CTL immunity derived from VSV immunization [32]. To address whether CD8+ mT-cell inflation influences late CD4+ T-cell-dependent CD8+ T-cell responses, we used an animal model of functional CD8+ mT-cell inflation previously established by our lab [33].
We found that CD8+ mT-cell inflation renders compromised CD4+ T-cell-dependent CD8+ T-cell immunity and identified the existence of T-cell anergy in naïve compartments [34]. This ovalbumin (OVA)-specific mT-cell inflation animal model was developed by transferring concanavalin-A (ConA)-stimulated monoclonal CD8+ T cells derived from OVA-specific T-cell-receptor (TCR) transgenic OTI mice into irradiation-induced lymphopenic C57BL/6 (B6) mice [33,34]. For this study, we also generated another two animal models with CD8+ mT-cell inflation. These included transferring, 1) ConA-stimulated monoclonal CD8+ T cells derived from LCMV glycoprotein (Gp)-specific TCR transgenic P14 mice, and 2) ConA-stimulated polyclonal CD8+ T cells derived from B6.1 (CD45.1 congenic) mice into irradiation-induced lymphopenic B6 mice to form a Gp-specific and a CD45.1-specific mT-cell inflation model, respectively. By using the Gp- and CD45.1-specific mT-cell inflation models, we assessed whether mT-cell inflation affects OVA-pulsed dendritic cells (DCOVA)-triggered CD4+ T-cell-dependent and recombinant OVA-expressing Listeria monocytogenes (rLmOVA)-induced CD4+ T-cell-independent CD8+ T-cell immunity. We found that CD8+ mT-cell inflation does not affect CD4+ T-cell-independent priming of CD8+ T-cell responses derived from rLmOVA infection but does reduce DCOVA-induced CD4+ T-cell-dependent priming of CD8+ T-cell responses [34]. We found that CD8+ mT-cell recall responses were not affected by CD8+ mT-cell inflation. However, we found that naïve CD8+ T cells isolated from the spleen of mice with CD8+ mT-cell inflation had a defect in cell proliferation capacity upon stimulation in vitro and in vivo. Furthermore, flow cytometric characterization of CD8+ T cells in the naïve compartment (CD44 low) revealed an upregulation of T-cell anergy-associated gene-encoding E3 ubiquitin ligase (Itch) and gene related to anergy in lymphocytes (GRAIL), which are well-known for rendering T cell defect [34]. Our finding is also supported by our another recent study that reported the expression of T cell inhibitory receptor, programmed cell death protein-1 (PD-1) in chronic infection and demonstrat6ed that CD40L signaling could overcome T cell exhaustion [35]. Therefore, our finding that CD8+ mT-cell inflation rendered compromised CD4+ T-cell-dependent CD8+ T-cell immunity via naïve T cell anergy may partly explain the susceptibility of the elderly to pathogens and may also suggest that costimulation and TLR signaling can be a potent strategy for rescuing of T cell anergy in elderly (Figure 1).
The immune system is involved not only in controlling malignancies and infections but also play an essential role in tissue homeostasis and repair [36]. Given that elderly have progressively decreased general immunity and immune responses to vaccination [37] Thus the prevention or compensation for age-related immunological defects is highly imperative for a healthy aging. The use of adjuvants in the elderly with CD8+ mT-cell inflation and compromised immunity could be one of the strategies to improve vaccine efficacy and overall immunity [38]. TLR ligands have been found to induce DC maturation and licensing, leading to CD4+ Th1 and effective CTL responses and are potent vaccine-adjuvant candidates for improvement of vaccine immunogenicity [39,40]. A cationic lipid–DNA complex (in Fluzone vaccine) has been shown to improve protection efficacy considerably in nonhuman primates against human seasonal influenza virus isolate [41]. A synthetic TLR4 agonist glucopyranosyl lipid adjuvant-stable emulsion combined with split-virus vaccine has also been found to boost T-cell responses to influenza vaccination, leading to improvement in vaccine-mediated protection against influenza in the elderly [42]. Our data demonstrated that recombinant bacteria (rLmOva), which is known to induce TLR signaling, was able to elicit primary CD8+ T-cell responses under mT-cell inflation. Our data suggested that TLR signaling can break naïve T-cell anergy under mT-cell inflation, which greatly supports the use of TLR ligands as adjuvants to enhance the vaccine efficiency in the elderly. Overall, our data revealed that CD8+ mT-cell inflation rendered compromised CD4+ T-cell-dependent CD8+ T cell immunity via naïve T-cell anergy, and thus show promise for the design of efficient vaccines for the elderly, who often show CD8+ mT-cell inflation.