Construction of Photonic Crystal Films for Chemical Sensing
A photonic crystals film (PCF) which consists of a porous structure with a highly ordered periodic arrangement of nanopores has been used for sensing mixtures of gasoline and ethanol (EtOH). Refractive index difference between the wall (silica) and air which occupies the empty pore results in the structural color of the PCF. This color disappears when the nanopores are infiltrated by a liquid with a similar refractive index to silica (or silicon dioxide). The disappearance of the structural color provides a means to construct a sensor to discriminate between various gasoline/EtOH mixtures based on their wettability of the nanopores in the PCF.
In this paper, an array of silica-based PCFs is synthesized on a silicon substrate with a precise control of nanopore properties using the co-assembly/sedimentation method. Using this method, we benefited from having different PCFs on a single substrate. Chemical coatings, neck angles, and film thicknesses on each PCF were the three factors used to tune the wettability of the pores. Nanopore wetting by gasoline/EtOH mixtures was studied in a systematic manner and combinations of the three factors were used to develop a sensor for differentiation of various gasoline/EtOH mixtures. The final developed sensor that consisted of an array of several PCFs was able to differentiate different gasoline/EtOH mixtures.
Keywords:Photonic Crystal Film; Structural Color; Nanopores; Gasoline/Ethanol Mixture; Sensor
Photonic crystals made of various compositions have been developed to be used as chemical sensors for sensing pH, solvent and etc. [1,2].
The refractive index difference between the matrix material (i.e. silica) and air (which occupies the empty nanopores) is related to the structural color of a photonic crystal film (PCF). The surface of the nano-sized pores inside the PCFs is typically coated by hydrophobic/oleophilic materials such as fluoroalkylchlorosilanes (FACS) [3]. These coating materials will cause the pores wetted or not wetted by certain liquids. When a liquid with a similar refractive index to the matrix wets the nanopores, the structural color disappears due to the refractive index matching between the liquid and matrix. The disappearance of the structural color provides a way to differentiate various liquids with different compositions based on the wettability of the nanopores [4-7].
In this study, we have created a sensor strip that consisted of an array of six PCFs by using an optimized combination of structure properties, thickness and surface coating. The sensor strip has been shown to have the capability to differentiate between different liquid mixtures (gasoline /EtOH mixtures) with a simple-to-read wetted/nonwetted platform.
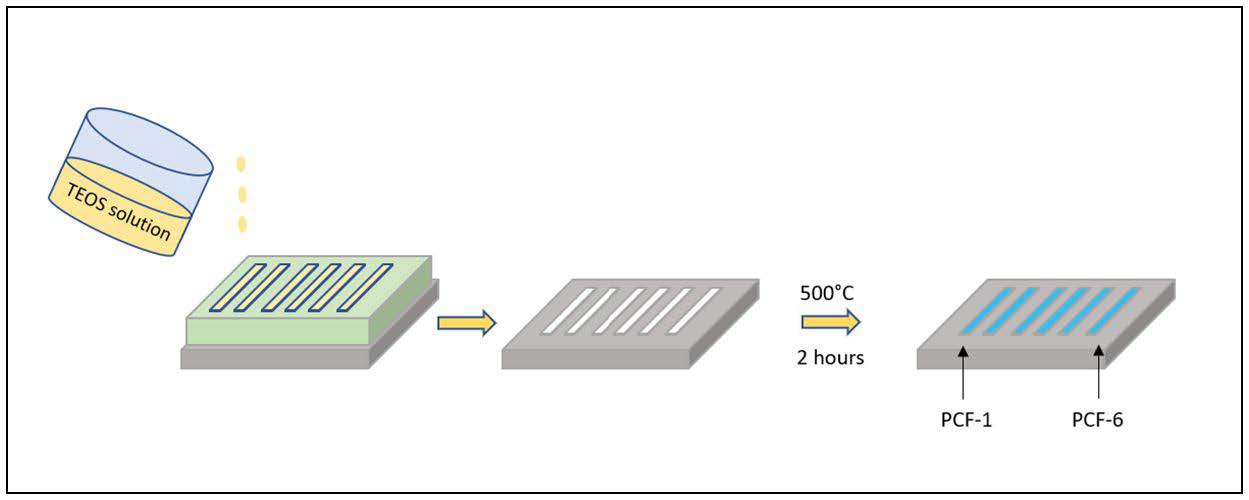
In order to synthesize silica-based PCFs, silicon (Si) wafers were cut into a rectangular strip (4 × 5 cm) using a diamond glass cutter. The Si substrate was cleaned in the piranha solution (70 mL concentrated H2SO4 and 30 mL 30% H2O2) for 15 min to produce a hydrophilic surface. Then, the substrate was rinsed with DI water. A mixture of 0.01 M HCl, tetraethylorthosilicate (TEOS) and anhydrous ethanol (EtOH) was prepared in a glass vial (a ratio of 1:1:1.5 w/w/w HCl/TEOS/EtOH) as the matrix. This solution was mixed thoroughly using a magnetic stirrer at 200 rpm for 1 h. Polymethylmethacrylate (PMMA) nanospheres (diameter: 318 nm) which are suspended in water (1-10% solid content), acted as the template. The PMMA stock bottle was sonicated for 30 min to homogenize the colloids. Aliquots of PMMA colloids (17 μL) and the matrix solution (1.4 μL) were added to a definite amount of DI water (750 μL) to form TEOS solution. They were then capped and sonicated for 1 h. After preparing the TEOS solution, it was deposited onto the Si substrate defined by a pattern using polydimethylsiloxane (PDMS) trenches of 20 mm of length, 2.5 mm of width and 1 mm of depth. The TEOS solution was left to be dried in a humid atmosphere (to decrease solvent evaporation rate).Then Si substrate, with the white-colored film deposited on its surface, was placed in a programmable oven, with the temperature ramped up to 500 °C over 4 h, held at that temperature for 2 h, and ramped down to room temperature over 1 h. This process led to thermal decomposition of the template (PMMA nanospheres) and calcination of the matrix (TEOS), forming the silica-based PCF. An array of several PCFs was formed on the same substrate. The PCFs were then coated with trichloro (3,3,3-trifluoropropyl)-silane (Figure 1 ).
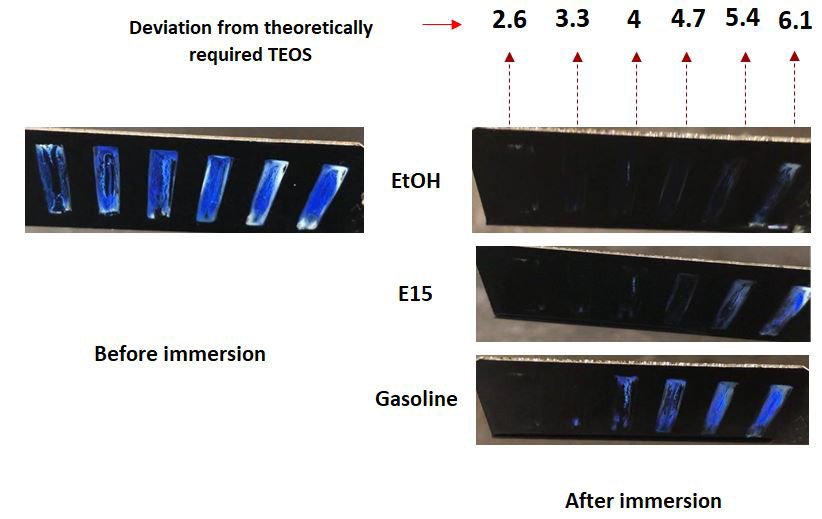
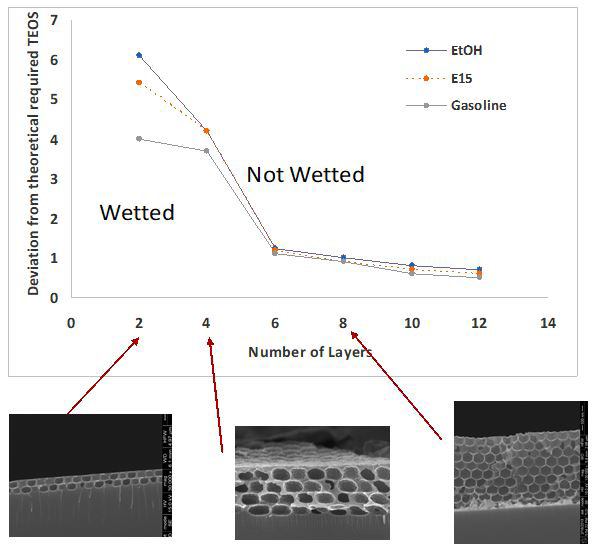
There are mainly three factors that can have effect on wetting behaviour of PCFs: structure properties (neck angle), PCF thickness and chemical coating which change the surface chemistry. We have used a combination of these factors and created six PCFs with different neck angles, and thicknesses. Figure 2 shows the results indicating how the PCFs differentiate between different gasoline/EtOH mixtures. Here pure EtOH, 15% ethanol in gasoline (E15) and pure gasoline are differentiated. The difference in wetting behaviour originates from different surface tensions of gasoline /ethanol mixtures.Figure 3 shows the graph of using different PCF layer number and deviation from theoretical TEOS to differentiate between different liquids. Here, the different liquid mixtures are differentiated successfully using two but not 6 or more layers.