Development of B1 Nested PCR for Assessing the Prevalence of Zoonotic Protozoan Disease Agent Toxoplasma Gondii among Food Animals from Karnataka State, Southern India
Toxoplasmosis is a pandemic zoonotic disease infecting wider range of warm blooded animals including man, marine mammals and birds. The present study was aimed for direct detection of natural infection of Toxoplasma gondii from edible portions or meat samples of different food animals such as sheep and goats, fishes and backyard chicken from the locally available mutton and fish stalls intended for the purpose of human consumption from North and Southern part of Karnataka State, India. This sampling was from unorganised stalls where muttons were sold by processing after slaughtering the animals, simultaneously. This study analysed using the total genomic DNA (218 numbers) extracted from 110 numbers of sheep, 34 numbers of goat (liver, heart and brain tissues), 41 numbers of fishes and 33 numbers of brain tissues of backyard chicken by B1 nested polymerase chain reaction (PCR) method, which showed an overall prevalence of 22.47% T.gondii infection among various meat and offal analysed, of which mutton and chevon revealed 29.09% and 38.23%, respectively. The results of the present study showing evidence that there is a higher prevalence of T.gondii infection among meat for human consumption, which may pose directly a threat to human beings. Further, the designed B1 nested PCR revealed 100% sensitivity and 100% specificity with the positive T.gondii DNA.
Keywords: B1 nested PCR; Chevon; Chicken; Fishes; Mutton; Prevalence; Toxoplasma gondii
Toxoplasma gondii (T.gondii) is an intracellular protozoa, which affects all warm blooded animals including man but cat is the definitive host, from which originally originating [1]. Worldwide, it infects almost one third of the human population. Due to its facultative nature, this has developed multiple ways of transmission among different host species [2]. Also, human and animals get affected to this infection by contaminated food or water with oocysts excreted into environment by the infected felids. Transmission of T.gondii takes place with different stages through infective oocysts or tissue cysts and tachyzoites found in either undercooked or raw meat or primary offal (viscera) of various food animals. Further, it may also happens with tissue transplants, blood products and unpasteurised milk containing tachyzoites, contaminated vegetables and water by oocysts [2-5]. Usually, infection with toxoplasma is asymptomatic in most of the immunocompetent [6 ] but with few individuals, it may lead to severe acquired conditions of chorioretinitis, pulmonary forms or life threatening if acquired through congenital route or as a sequelae of reactivation in immune compromised individuals [7-10]. In some cases, this infection may lead not only abortion but destructive inflammation including the brain and eyes [9]. A large fraction of ocular toxoplasmosis is common in human beings [11,12].
The first report of T.gondii in marine mammals (harbour seal) was reported by Van Pelt and Dietrich followed with several other pinnipeds [13,14]. Moreover, it also causes severe infection among marine mammals, which leads to morbidity and mortality, due to the contamination and survival of oocysts in sea water [15-17].
According to a nationwide study conducted by the Office of Registrar General and Census Commissioner (ORGCC), 71% of people are non-vegetarian while 28.85% are vegetarian in India; further the Telangana has highest number of non-vegetarian to 98.7% followed by West Bengal with 98.55%, Odisha with 97.35% and Kerala with 97% [18]. According to the Sample Registration System Baseline Survey in the year 2014 (central government of India), Karnataka stands in 11th place in the country in non-vegetarianism with 79.1% men and 78.1% women being non-vegetarians [19]. Therefore, it is important to undertake this study to detect the presence of this important zoonotic infection among different meat samples, which are subjected to human consumption wholly. Due to the false positives of serological technique and its tedious procedure, nested polymerase chain reaction (n-PCR) technique is the preferred method for the detection/diagnosis of toxoplasma infection. It has the advantages of higher sensitivity and specificity and more cost-effective for the detection of toxoplasmosis [20,21]. Above all, mouse bioassay method is the main confirmatory test for T.gondii detection and diagnosis, but it is a laborious and time-taking technique, it is not very suitable for routine monitoring activities on higher number of samples and also from an ethical point of view (European Food Safety Authority) [22,23]. One such study dealt about the isolation of viable T.gondii from each numbers of 2094 of meat, chicken and pork received from 28 various geographical areas of the United States [24].
In human beings, T.gondii prevalence varies from 15.0% to 85% depending on social habits, geography and climatic conditions and even up to 95% of incidence shown in some populations in various places around the world whereas the incidence of animal toxoplasmosis is highly variable among various important livestock species with the global prevalence rate of 28.9% in goats, 38.5% in sheep, 66.0% in cattle, 73.0% in dogs and 84.1% in cat [1,25-29]. In India, it varies from 8.8 to 37.3% (average 22.4%) based on serological survey and 2 to 24.3% in human beings whereas in animals it has been recorded as low as from 2% to 100% in different animal species and in backyard chicken (Gallus domesticus) with 20.33% and 39.5% [30-38]. Since the stray cat populations are very high in the country, it may contaminate the environment with millions of oocysts, therefore, the chances of ingestion of oocysts expected to be high by the grazing animals and also contracted by human through food, water [1]. Mutton, chevon and fishes are the important sources of protein in India, thereby acts as a potential mode of transmission of T.gondii to humans [15]. But in India, there is no report of T.gondii prevalence among fish populations. Backyard poultry is one of the important species, which are act as the surveillant to check the environmental contamination by detecting the presence of oocysts of T.gondii and are the important sources of transmission of this infection due to their habit of ground feeding, which contained the oocysts shed by the cats. Fewer sporadic studies have been reported on the presence of T.gondii from animals, chicken and AIDS infected patients but lack a data on meat, backyard chicken and fishes subjected for human consumption, which are collected from the local markets [34,35,39,40]. Therefore, the present study has been designed to detect and assess the prevalence of the natural T.gondii infection with newly designed B1 nested PCR among different meat samples from the selected regions of Karnataka state.
Mutton, chevon samples from sheep and goat and muscle portion of fish samples were collected from locally available mutton and fish stalls in and around Mysore (Southern Karnataka), which are unorganised sector, where slaughtering and processing of meat cutting takes place simultaneously for the purpose of human consumption. Similarly, the samples of brain tissues from backyard chicken have been collected from Bidar and Gulbarga (Northern Karnataka). Sampling details have been given in Table 1.
Brain samples from the backyard chicken were collected from the month of June 2016 to March 2017 from the regions such as Gulbarga (13 numbers) and Bidar (20 numbers), Northern Karnataka from different private poultry meat shops. The backyard chickens were raised under open free range conditions and brought to local market for meat consumption. The other food animal samples were collected from sheep, goats (small ruminants) and fishes (rohu, rui, or roholabeo-Labeorohita) from the month of February to April, 2017 from Mysuru city (Southern Karnataka). These samples were collected from different private mutton and fish stalls within the city area. Samples of hearts, liver and muscle portions weighed approximately of 50 gms were collected from each animal, placed individually in a small collection bag and brought immediately to laboratory, where it was kept at -20 °C for molecular biology work, according to Bacci, et al. (2015).
Overall, the total numbers of tissue samples (liver and heart) were collected from sheep (110) and goats (34), and muscular portion of fishes (up to 50 gms) were collected from retail mutton stalls, from different localities of Mysore city and brain tissues from backyard chicken from Gulbarga (13 numbers) and Bidar (20 numbers) region from Northern Karnataka. Collected tissues were stored at ice cubes and transferred to laboratory. These samples were preserved at -20 °C before further use.
The Indian State of Karnataka is located 11°30’ North and 18°30’ North latitudes and 74° East and 78°30’ East longitude. It is situated on a tableland where the Western and Eastern Ghat ranges converge into the complex, in the western part of the Deccan Peninsular region of India. The climate of Karnataka witness with arid to semi-arid in the plateau region, sub-humid to humid tropical in the Western Ghats and humid tropical in the coastal plains. Northern part of Karnataka is arid and receives only 711.5 mm of average rainfall per annum whereas the southern Karnataka is a semi-arid region, which receives 1286 mm rainfall per annum. The average high temperature during summer is 34 °C across the state. The average day temperature is 29 °C in the monsoon season. During winter temperatures range from 32 °C to below 20 °C.
The total genomic DNA from tissue samples of heart, liver, brain, and muscle were extracted as per the method described by with minor modification [41]. The different tissue samples such as brain (backyard chicken), heart, liver, and brain (small ruminants), and muscular tissues (fishes) were approximately weighed to 20-25 mgs out of 50 gms into 2 ml eppendorf tube and minced with cleaned scissor for betterment of digestions with Proteinase K as per the protocol described elsewhere. 1/50 volume of Proteinase K from Himedia (20 mg/ml, W/V) was added into the minced tissue sample and incubated at 65 °C for overnight with intermittent shaking of the sample. By adding further of 1/10 volume of potassium acetate (5M), incubated in ice for 30 min before centrifugation at 10000 rpm for 10 min. After transferring the supernatant into fresh eppendorf tube (2 ml), equal volume of phenol: chloroform and isoamyl alcohol (25:24:1) added and mixed several times by inverting tubes. Further, it was subjected to centrifuge at 5000 rpm for 5 min. The upper aqueous phase containing nucleic acid was transferred into new tube and added equal volume of chloroform and mixed it by inverting for few times. It was further subjected to centrifuge at 5000 rpm for 5 min. The aqueous phase was transferred with care in to a new eppendorf and added 2 volume of ice cold 95-100% ethanol and then inverts it several times. This has to be kept at -20 °C for 15 minutes to precipitate DNA. Later, it was subjected to centrifuge at high speed to collect DNA at 14000 rpm for 8 min. Further, pour off the ethanol and the remaining DNA pellet was allowed to dry partially and was further suspended in 0.5 ml of Tris EDTA (TE) buffer (pH 8.0) for using as template for PCR assay. The extracted DNA was stored in aliquots at -20 °C. The purity and concentration of isolated genomic DNA was checked by 0.7% agarose gel electrophoresis.
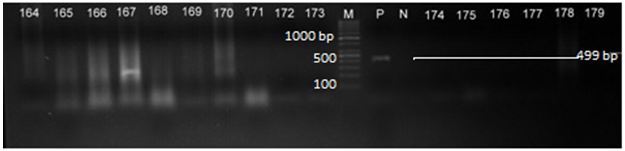
B1 gene primers were designed using NCBI collected T.gondii B1 gene partial sequence having 2214 bp (accession no. AF179871) of length was used for the present study. The primers used in this study have been mentioned in Table 2. After standardisation of PCR, the reaction was carried out in a mixture containing 4.0μl of genomic DNA as temple, 1.0μl of each forward and reverse primer (20 pmoles of each primer), 2.0mM magnesium chloride, 200 μM of each deoxyribose nucleic acid (dNTPs), 10x PCR buffer with 1.0 U of Taq DNA polymerase (MBI Fermentas, Bangalore) and made up with distilled water to the final volume of 25 μl reaction. The PCR reaction was performed over 30 cycles in Simply Amp thermocycler (Applied Biosystems), using the following cycling conditions. The cycling conditions of primary PCR were programmed with first series of thermal cycling (pre-PCR) consisted of initial denaturation at 94 °C for 10 min, followed by 30 cycles of denaturation at 94 °C for 90 sec; annealing at 57 °C for 1 minute 30 seconds and extension at 72 °C for 2 minutes. Final extension was performed at 72 °C for 8 minutes. The PCR product is diluted 1:25 with milli Q distilled water (Millipore water, India), which was used as template for nested PCR with the thermal cycling consisted of initial denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 45 sec; annealing at 56 °C for 1 minute and extension at 72 °C for 2 minutes. Final extension was performed at 72 °C for 7 minutes. Every reaction was included with one each of negative control with milli Q water and positive with PTG strain of T.gondii, which was a kind gift by Dr. Su, Associate Professor, University of Tennessee, USA. The amplified product was size matched with 100 bp DNA ladder (Thermo Fisher Scientific). A part of the nested B1 PCR amplification has been shown in Figure 1. The result of the PCR was documented in the UV gel documentation systems (Bio-rad USA).
The sensitivity and specificity of B1 nested PCR primers were carried out with positive PTG strain of T.gondii. The designed primers could able to detect T.gondii DNA with 100 percent sensitivity and 100 percent specificity. Species specific total genomic DNA isolated from buffy coat from blood of sheep, goat, fish and chicken revealed no amplification of nuclear DNA except T.gondii specific total genomic DNA. The sensitivity of the PCR was tested with 5x10-2 pg, 0.1pg, 1 pg, 10 pg of purified T.gondii parasite DNA (PTG) as per the method used by previous authors [42].
The B1 gene copy was used to amplify the target DNA of T. gondii parasite. The extracted total genomic DNA from all the samples (218 numbers) were subjected to the check for the purity using UV-VIS Spectrophotometer (Perkin-Elmer, USA), where the OD showed the purity of greater than 1.7, which was further subjected to PCR with B1 specific primers. With positive PTG DNA having the sensitivity and specificity of 100 percent with the designed primers, the samples were subjected to nested PCR for the direct detection of T.gondii specific DNA.
The present study was investigating directly for analysing natural T.gondii infection from different food animals such as sheep, goats (small ruminants), and fishes from in and around Mysore city (Southern Karnataka) and backyard chicken from Gulbarga and Bidar region (Northern Karnataka). The collected samples were liver, heart and brain tissues from sheep and goats, muscles from the fishes and the brain tissues from the backyard chicken. The total genomic DNA extraction was carried out using salting method with minor modification. A total of 218 extracted genomic DNA samples were analysed by B1 PCR for the presence of T. gondii. The B1 PCR primers were successfully designed from the NCBI collected full length sequence (accession number: AF179871) to amplify the 1484 bp and 499 bp amplicon, which is a repetitive 35 fold gene copy to target the DNA of T.gondii specific for the diagnosis and detection. Samples of mutton/offals collected from sheep revealed 32 positives out of 110 numbers of samples analysed, which revealed an overall prevalence of 29.09% among all samples analysed by B1 nested PCR. The details of B1 nested PCR detection from different samples have been mentioned in Table 3 whereas the case of chevon of goat examined, a total of 13 positives were detected out of 34 numbers of samples examined, which revealed an overall percent prevalence of 38.23% and further details of the detection have been mentioned in Table 3. Altogether, the percent prevalence of T.gondii among small ruminants from Mysore city revealed 31.25%. In the case of fish populations, only 2 positives were recorded out of 41 muscles examined with a percent prevalence of 4.87% whereas in the backyard chicken collected from Bidar (20 numbers) and Gulbarga (13 numbers), it revealed only 2 positives out of 33 number of brain tissues analysed with a prevalence of 6.06% from Bidar and Gulbarga region (Northern Karnataka). The B1 nested PCR revealed an overall detection of 49 positives out of 218 samples analysed, which showed an overall per cent prevalence of 22.47% of infection among different food animals examined (Table 3).
The present study was aimed to assess the qualitative detection and the prevalence of this important zoonotic parasite, T.gondii from different meat samples collected from sheep, goats, fishes and backyard chicken. Meat is the foremost important source of this T.gondii infection to human beings by consuming raw, undercooked or improperly cooked meat/primary offal (viscera) [2]. Sheep, goat (red meat), chicken (white meat) play an important alternative protein supplement and fishes provide cheap-protein rich food to the people in Indian subcontinent [43]. However, data is lacking for the prevalence of this zoonotic parasitic infection.
For T.gondii DNA detection, tissues of the heart is mostly preferred sampling because of commonly infected site but at the same time, viable tissue cysts of T.gondii prevailed in almost most of edible portion of a particular animal, which remain viable for many years in food animals [44-46]. Therefore, heart, liver, brain and muscular portions were utilised for this study. This present study revealed 49 (24.31%) positives out of 218 numbers of total edible meat samples collected from retail meat stores/open market analysed by nested B1 PCR. Out of 49 positives (22.47%), samples of sheep showed 32 positives (29.09%) (19/33 no. of liver; 4/27 heart; 8/43 no. of liver and heart; 1/7 brain examined) whereas goat revealed 13 positives (38.23%) out of 34 examined (2/8 liver; 3/8 heart; 6/13 liver and heart; 2/5 brain examined); altogether from small ruminants, it was recorded 45 positives (31.25%) out of 144 samples examined. The brain tissues of backyard chicken revealed 2 positives (6.06%) out of 33 examined whereas the muscle portion of fish revealed 2 positives (4.87%) out of 41 examined. This study revealed higher numbers of positives from goat and sheep meat as compared to the studies previously published. One such study conducted from slaughter house of sheep and goats from Chennai city revealed positivity of 3.67% and 3.50% respectively out of 193 numbers of tissue sample subjected to B1 PCR with published primer with PCR amplicon of 193 bp [47]. Another recent study conducted on slaughtered sheep and goat from 400 numbers of cardiac/skeletal muscle tissues from Punjab, Uttar Pradesh and Chandigarh States from Northern India revealed an overall prevalence of 1.5% positives with 1.69% and 1.34% from sheep and goat, respectively by B1 based self-designed primers with PCR amplicon of 580 and 531 bp length [36]. Earlier study showed lower antibody titre from the sheep reared under intensive system than the sheep managed by semi-intensive [48-51]. This indicate that the seropositivity has direct correlation on the management in the Western countries. Whereas in Indian subcontinent, almost 100% of sheep and goats are being reared under free range system. Thereby, the possibility of getting infection with oocysts must be high due to the excreted toxoplasma oocysts remains infectious for a longer duration. The goat revealed higher percentage of positives (41.17%) as compared to sheep (35.54%), which is contrary to the study conducted on viable T.gondii isolation from small ruminants [47,52].
Few other studies conducted outside India on various samples from small ruminants, chicken and fishes based on B1 PCR have been documented. B1 PCR used to detect T.gondii DNA from milk of sheep (345 no.) and goats (280 no.) from North-West region of Iran revealed the prevalence of 3.04% (19 positives), of which 4.63% sheep (16 positives) and 1.07% goat (3 positives) milk samples [53]. Another recent study from the same region of Tabriz (North-Western Iran), from meat samples (150 numbers) including chicken (50 no.), beef (50 no.) and lamb (50 no.) were revealed an overall prevalence of 17.33% (26 samples) positives for T. gondii including 8% (4 no.) of chicken, 16% (8 no.) cattle and 28% (14 no.) of sheep by B1 PCR with the product length of 194 bp [54]. Further, samples from sheep of Southern Iran revealed 34.32% infection by ITS-1 based study [55]. Whereas in the case of milk samples of goat, from the infection was found to be 7.8% in Tunisia, 65% and 43% by B1 based real time PCR and nested PCR in Poland and also 13% of blood and milk samples were positives by nested PCR from 60.6% MAT positives [56-58]. A recent study revealed a higher prevalence (79.0% from 105 numbers) of T.gondii infection from free range chicken subjected to direct detection of T.gondii DNA by nested B1 PCR (529 bp) in Thika regions, Kenya [59]. Another study explained about the two groups of broiler chickens reared under two different conditions revealed positives of 8 (50 numbers) and 35 (80 numbers) by B1 PCR (133 bp), respectively [60]. Another authors investigated the occurrence of T.gondii from eight different farms (altogether 40 numbers) in neighbouring areas to the Pantanal in Nhecolândia, MatoGrosso do Sul (Brazil) and DNA analysis of the pooled samples of blood, brain and heart showed 40 % (16 positives) prevalence for T.gondii by B1 based PCR, which was compared with MAT assay, in which 5.2% of PCR negative were positive by MAT [61]. Another study indicated that PCR can be used along with ELISA for confirmatory test for the diagnosis of toxoplasmosis in ostriches birds, in which B1 PCR (231 bp) revealed 7.5% (9 positives) T.gondii infection from 120 blood samples examined [62]. A study based on blood samples collected from local breed chicken and aborted woman revealed 16% and 24% positives with highly specific B1 gene specific real time PCR [63]. These differences in the prevalence might be due to the differences in cat densities, the number of chickens examined and sanitation conditions in those areas [64].
The present B1 nested PCR applied on muscle tissues of fishes revealed 4.87% (2 positives) of T.gondii infection from a total of 41 numbers examined. As far as our knowledge is concerned, this study on fishes could be the first one in India. This study was partially in agreement with the study conducted by Aakool and Abidali, where in a total of 96 samples of muscle (24), liver (24), enteric (24) and gills (24) of native freshwater fish Cyprinuscarpio subjected to Real time (RT) PCR to detect B1 gene (94 bp fragment) of T. gondii, revealed 16 enteric and 2 gills samples found positives while other samples showed negative results. This finding of T.gondii positivity from muscle tissues of fishes are further substantiated by the finding revealed by Sanders, et al., who had used Zebra fish (Danio rerio) as a model for T.gondii establishment, in which the intra peritoneal inoculation of two strains of T.gondii (10 tissue cysts/fish) could able to form a pattern of infection, which was similar to the one found in mammalian infection with parasites developing in the somatic muscle including the different internal organs. Therefore, this infection might be acquired either from contaminated environment or due to consuming filter concentrate invertebrates [65-67]. Some studies in human suggest that the nested PCR can be valuable in comparison with serological methods, where in one such study based on B1 PCR showed positivity in 67.8% (139 numbers of positives) of diabetic patients out of 205 examined whereas other authors revealed that 41% (56 positives) of patients infected with T.gondii than ELISA wherein IgM-ELISA detected 6.5% (9 positives) and the IgG-ELISA detected 38.6% (53 positives) of the patients, respectively [68,69].
The numbers of backyard chicken samples revealed only lesser percent prevalence (6.06%) of T.gondii infection in the present study. There is no studies available in India from backyard chicken samples based on nested B1 PCR except a few serological study revealed a prevalence of 39.5% T.gondii infection out 185 chicken collected from slaughter markets in and around Madras City and 17.9% from a total of 741 chickens examined from different places from Maharashtra and Tamil nadu (central and south India) [38]. Out of these, five T.gondii free cats fed with seronegative heart and or brain tissues of chicken shed oocysts, indicating that a seronegative samples showed positivity of T.gondii infection [39]. Further, the lesser percent in the present study could be due to limited samples confined to two regions viz., Bidar and Gulbarga (Northern Karnataka). These results indicating that these differences in prevalence values might be due to the quantum of oocysts shedding from the infected cats into the environment, different susceptibility of breed to the infection, rearing conditions, and climatic situation and finally the oocysts are known to survive longer in moist soil from months up to years [1,70].
Therefore, it has been concluded that this study revealed a moderately higher percent prevalence of T.gondii infection among different food animals intended for human consumption. Therefore, handling of meat or meat producing animals should be done carefully. Further, to ensure not getting infection, proper cooking is necessary before consumption. Further, the designed B1 nested PCR detection method could able to detect T.gondii more precisely for the screening of large numbers of samples for epidemiological studies. Further, studies are required with more sampling to know the detection limit with other serological diagnostic methods for the missing samples if any.
We would like to thank the Director, Defence Food Research Laboratory, Mysore for his excellent support for providing facilities and support for this study, which was funded by DRDO, Delhi.