Differential Expression of Serum LDH Isozymes in the Fish Labeo rohita as a Function of the Pesticide Carbamate
Lactate dehydrogenase LDH (E.C. 3.1.1.27) is one of the chief enzymes of carbohydrate metabolism which catalyses the oxidation of lactate and reduction to pyruvate in glycolysis. It is a dimeric molecule consisting of two separate loci which code for A and B sub- units of the enzyme. The A and B sub- units associate to form 5 tetrameric isozymes (A4, A3B1, A2B2, A3B1 and B4). Isozymes are multiple forms of single enzyme which have different isoelectric points and therefore they can be separated electrophoretically. Following the treatment of carbamate there was an induction of oxidative stress in the experimental fish which is one of the mechanisms of action of carbamate. There were 3 bands in the control fish having LDH A (55.6%) (LDH A’ absent), LDH B (31.3%) and LDH B’ (13.1%). Following the treatment of carbamate, there was a differential percentage in increase or decrease of different serum isozymes of LDH in the fish, Labeo rohita. It was interesting to observe that following the treatment of carbamate, the B subunits increase till 96h in contrast to the treatment of methyl parathion where the A subunit increased during 24h as earlier reported by Ray & Sinha in 2016. The physiological and biochemical significance are reported herein for the first time.
Keywords: Lactate dehydrogenase isozymes; Labeo rohita; Carbamate
Pesticides are known to adversely affect a number of biological functions, thus causing harm even to the non- target organisms. Organophosphate and carbamate compounds are known for its persistence in the environment and accumulation in the tissues for long periods for controlling the loss of produce due to the pest attack and as a consequence of the demand for producing more food, there has been an increasing use of pesticides in the developing countries [1,2]. Many pesticides have been reported to produce a number of biochemical changes in fish [3-7]. It may provide an early warning signal in stressed organism. The sources of these parameters are the indicators responding to environmental effects and can also serve as biomarkers for the xenobiotic exposure. The toxic effects of pesticides on non- target organisms in the environment can be studied by monitoring [8]. The analysis of biochemical parameters in fish can contribute to the health status of the animal and also environmental habitat conditions, pesticides have been shown to cause alterations in the activities of many enzymes concerning to cellular metabolisms.
Carbamate, an extremely toxic insecticide, is known to produce toxic manifestations of hypercholinergic activities involving central as well as peripheral organs by inhibiting acetylcholinesterase (AchE) enzyme at synapse in the brain and neuromuscular junctions [9-11]. In addition to AchE inhibition, several other metabolic derangements are also expected to occur like energy metabolism of Creatine kinase (CK) and CK isozymes actually poisoned with carbamate in rats [12]. Enhanced activity of CK has been reported in fish following the treatment of methyl parathion [13]. The different CK isozyme subunits (Ck-M and Ck-B) were differently expressed in the serum of Labeo rohita. Although Ck-M was predominant in skeletal muscle but Ck-B was expressed to a great extent in the heart following the treatment of methyl parathion.
Lactate dehydrogenase (LDH), another cytoplasmic biomarker enzyme, plays an important role in glycolytic cycle and is therefore crucially important for normal muscle physiology. The activity of LDH is expected to be altered by carbamatetoxicosis for 3 specific reasons: a) increased membrane permeability, b) direct effect of carbamate and its metabolites and c) effect of stress.
The activity of Ck is mainly present in the brain, heart and skeletal muscles whereas the activity of LDH is present in all tissues, with the liver, skeletal muscle and the heart having the highest concentration. In most cases of tissue damage, whether from disease process or by a chemical toxicant, the activity of Ck and LDH are reported to be enhanced and the degree of increase in the activity of such cellular enzymes depends primarily on the magnitude and severity of cellular damage [14,15].
LDH in fish has always been a subject of much attention but not much information is available with the changes in the expression of LDH isozymes on the toxicity of carbamate. The aim of the present paper is to evaluate the expression of LDH isozymes in the serum of fish, Labeo rohita after the exposure to sub- lethal concentration of carbamate for 96h using fully automatic clinical electrophoresis and densitometer.
Presently, we do not have any Ethical Committee in our University. But however, we have followed the ethical norms, which are followed elsewhere which is evident in the Materials & Methods Section.
The experiments were repeated several times and only arithmetic mean of the experiments at each concentration was taken to express the results. LC50 values were determined by EPA Probit analysis program [16]. The 1/3rd of LC50 of the pesticide carbamate was 14.0 ppm for Labeo rohita.
Labeo rohita, a common carp, was obtained from the local hatchery. Fishes were acclimated to laboratory conditions for about 5-7 days. They were kept in aquarium tank (250 L) and water was constantly aerated by a static system. During the acclimation period, they were given artificial (commercial) feed composed of ground shrimps available in the local market to avoid the possible effects of starvation. The feeding and maintenance of the fishes and physico-chemical characteristics of the aquaria water were measured (Table 1). Short-term test of acute toxicity over a period of 96h were performed on the fishes following the renewal of bioassay. The fishes were exposed intracoelomatically with 1/3rd of LC50 of the pesticide carbamate. After 24, 48, 72 and 96 h of exposure fishes were sacrificed and the serum LDH isozymes were studied
The fishes were taken out of the aquarium water individually through fish net with a minimum possible disturbance. After preliminary investigations, the blood samples were collected from caudal fin as described by many authors. In the present study, the blood collection from caudal fin had to be abandoned because there was an unusual elevation in Lactate dehydrogenase (LDH) and Creatine phosphokinase (CPK) activities which were recorded due to leakage from the surrounding muscle tissues. Thus, cardiac sampling was the only suitable method available as an alternative to obtain blood under the present study. After the blood collection, the serum were separated and processed for LDH isozyme study.
The statistical analysis of the results was made using a statistical software package Systat v. 7.0 (Spss. Inc., Michigan, Chicago, USA). The results were expressed as Mean ± S.D. The sample size in each group was 5 (n=5).
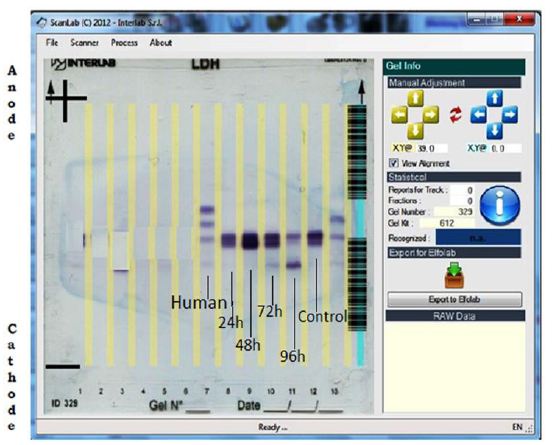
Fully automatic clinical electrophoretic unit is a system software controlled electrophoretic separating system having a voltage, current and runtime control, operated by an inbuilt program present in the driver software of the instrument. Fully Automatic Clinical Electrophoresis Model:- Interlab- Pretty was used for the present study having software namely “Interlab”. The system constituted of an automatic dispensing system for example, a Teflon coated Peltier Plate for placing previously casted agarose gel over sterile plastic plate having bar- code and valve- compressor system for pouring appropriate amount of staining, destaining and washing solutions over the gel plate after the completion of the migration. About 30μl of previously separated unhaemolyzed serum were placed in the sample space and loaded over the gel plate by dispensing system to achieve single straight line of application. The temperature for migration was set at 29 °C,time for migration was 5 mints, voltage for migration was 400V. Post migration secondary treatment of the gel was needed, performed in an another chamber with an incubation period of 30 mins. At 45 °C after addition of staining solution. The staining solution contains pyruvate and nitro-blue tetrazolium (NBT). NBT was reduced to formazan by NADH, also present in the staining solution which stained LDH isozyme bands. The fixation of the bands was achieved in the above mentioned external chamber. After staining and fixing the bands, the gel plate was replaced into the electrophoretic chamber for repeated destaining by a destaining solution containing 5% acetic acid (v/v). Then washing was done with a surfactant solution for a number of times previously programmed in the software. Following the completion of the steps, scanning of the gel plate was done in a densitometer with driver software “Scanlab” provided by the manufacturer.
To draw conclusion from the bands formed by the LDH isozymes, human serum LDH isozyme electrophorogram was used as a reference as the system software was standardized for the human serum LDH isozyme band formation.
A set of fish untreated with pesticide carbamate was taken as control and simultaneously treated with carbamate for 24, 48, 72 and 96h. They were maintained in separate aquaria exactly similar to their treated counterpart without being exposed to the pesticide carbamate intracoelomatically. The control set for each 24, 48, 72 and 96h were taken as negative control.
In general, any stress inducing substance affects the respiratory metabolism of an animal. Any alteration in the intermediary metabolism due to stress is bound to affect the oxidative enzymes like lactic dehydrogenase (LDH) and succinate dehydrogenase (SDH). Both enzymes are involved in carbohydrate metabolism and have been used as an indicative criterion of exposure to chemical stress. Animals respond to stress by activating a wide array of physiological and behavioral responses known as stress response. In response to stress, corticotrophin releasing factor (CRF) plays a key role by regulating the hypothalamic pituitary interrenal (HPI) axis which initiates a cascade of events that culminate in the release of glucocorticoids from adrenal cortex. Plasma cortisol is widely used as a general indicator of stressful conditions in the fish in which the body of fish emits immediate responses recognized as primary and secondary responses. The primary response is perception of an altered state by central nervous system (CNS) and the release of cortisol and catecholamines into the blood stream. Secondary responses occur as a consequence of the released stress hormone causing a change in the blood and tissue chemistry e.g. an increase in blood glucose. Cortisol activates glycolysis through the increase in LDH isozymes activities and modulates the cardiovascular functions. The whole process increases the glucose content to produce enough energy which may be required in a short period of time [17-19]. Earlier, the authors have also reported in the increase in CK isozymes in the fish Labeo rohita following the treatment of methyl parathion.
Lactate dehydrogenase (LDH) catalyzes the oxidation of lactate and reduction of pyruvate during anaerobic respiration. It is a tetrameric molecule consisting of two separate loci which code A and B subunits of LDH. The A and B subunits indiscriminately associate and form 5 tetrameric isozymes (A4, A3B1, A2B2, A1B3 and B4). Isozymes are multiple forms of single enzyme which often have different isoelectric points and therefore can be separated by electrophoresis. LDH electrophoresis patterns could help in investigating and diagnosing the pesticidal stress.
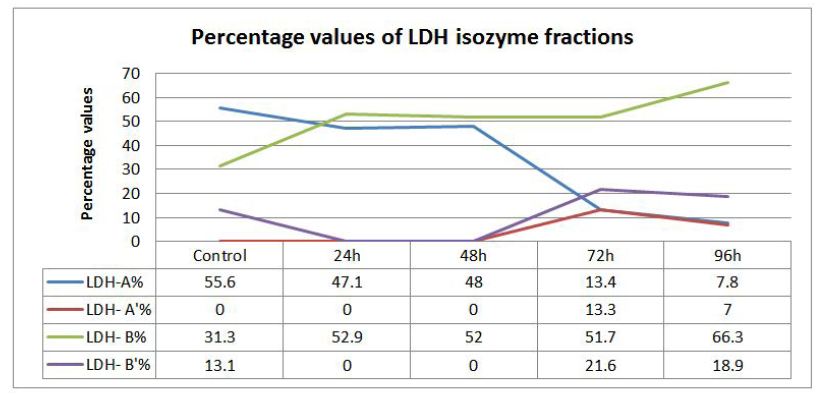
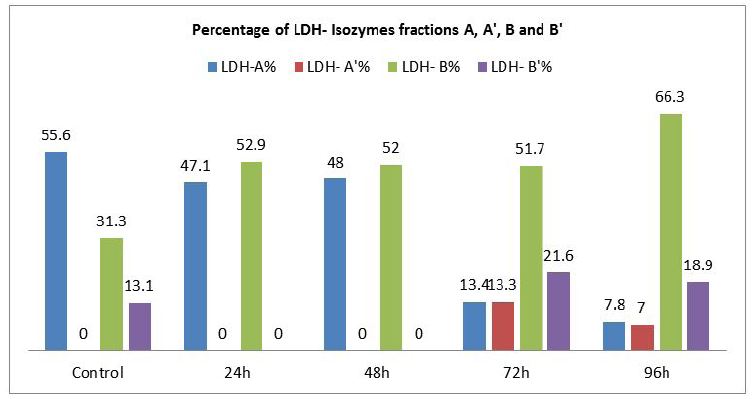
The present investigation was intended to evaluate the changes in the profile and characteristic patterns of LDH isozymes in the serum of the fish, Labeo rohita after sub-acutely treated with carbamate pesticide. The calculated values of LDH isozymes and percentage changes are given in (Figure 3). LDH A is the first of the five isoenzymes of LDH from cathode to anode (Figure 1). It is well known that LDH is present in the cells of every body tissues and in the case of damage; it is released from the cells to the blood. The normal functioning of the cell membrane is highly efficient at retaining the intracellular enzymes presumably by active process involving the general energy metabolism of the cell. Authors have already reported earlier that LDH is a highly sensitive biomarker but not specific biomarker. Separation and quantification of the five LDH isozymes provide a more informative and diagnostic tool than a single enzyme. This has been exemplified by the increase in the concentration of more anodic isozyme in the present study (Figure 3). It has been reported that during myocardial infarction anodic isozymes increase whereas, cathodic isozymes increase in the skeletal muscle dysfunction [20].
In the present investigation anodic isozymes increase suggesting carbamate may be affecting the heart more than the liver. It has been reported earlier that cathodic isozymes increased in the methyl parathion treated fish Labeo rohita suggesting muscular dysfunction (Figure 2). Figure 3shows that LDH electrophorogram consists of 4 bands from cathode to anode (Figure 1 and 3). In the control fish, LDH- A is 55%, LDH- A’ is absent whereas LDH- B is 31.3% and LDH- B’ is 13.1% (Figure 3). But following the exposure of carbamate LDH- A decreased to 47.1%, 48%, 13.4% and 7.8% in 24, 48, 72 and 96h respectively. LDH- A’ is absent in 24 and 48h but present during 72 and 96h with a concentration of 13.3% and 7.0% respectively. Whereas, LDH- B increased by 52.9%, 52.0%, 51.7% and 66.3% in 24, 48, 72 and 96h respectively. LDH- B’ is absent in 24 and 48h but 21.6% and 18.9% present in 72 and 96h respectively.
The differential percentage of increase/or decrease of different LDH isozymes in the carbamate exposed fish during different periods may be due to the differential mode of action of carbamate in different tissues. Secondly, gene duplication as well astranslational processing is being proposed as important factors in modulating tissue specific enzymes [21 ]. LDH- A’ is missing in the control fish as well as in the exposed fish except in 72 and 96h (Figure 3). The increase/ decrease/ absence of some LDH isozymes could be interpreted as a result of mutational events that would have occurred in the regulatory genes which could lead to the inhibition, alteration or constitutive gene expression. This is the reason why some bands of LDH isozymes became more intense or faint (Figure 1).
It was interesting to observe that LDH- A subunit increased in the fish, Labeo rohita following the treatment of methyl parathion suggesting muscle dysfunction but on the contrary carbamate treatment LDH- B subunit increased suggesting cardio vascular dysfunction. Thus LDH isozymes can be regarded as biomarkers of pollution.
This study suggests that alteration in the LDH isozymes activities may provide insight of the toxic injury and revealwhich tissues or organs are involved. The study also reveals that LDH isozymes activities in the blood serum may serve as useful quantitative in- vivo biomarker for ecotoxicological studies in animals.
The authors are grateful to DBT, Govt. of India for financial assistance for the project (Sanction Letter no. BT/PR/4762/BCE/8/903/2012; Dated: 11/3/2013). The authors also thank the Head of the Department of Zoology for extending all logistic support.