Flexible Micro Supercapacitor Based on Laser Scribed Graphene (LSG)
In the present work, graphene powder has been synthesized by laser scribing method. The resulted flexible light-scribed graphene is very appropriate to be utilized as micro-supercapacitors .Graphene conducts electricity and heat better than anything else and has combination of unique optical and mechanical properties. Electrons have nobilities in graphene over a hundred times those in silicon. In the present work we used a standard optical LightScribe DVD drive to directly do the laser reduction of graphene oxide (GO) films to graphene. GO was prepared by the modified Hummers’ method as reported elsewhere. Briefly, 2 g graphite powders were added to a mixture of 1 g NaNO3 and 46 ml H2SO4 and the mixture was cooled to 10 °C using an ice bath. In the next step, 6 g KMnO4 was gradually added to the solution and the reaction temperature was maintained below 20 °C. The mixture was then stirred at 35 °C for 2 h. The resulting solution was diluted by adding 92 ml of deionized water until a dark brown suspension was obtained. Then, the solution was treated by adding 340 ml H2O2 solution. Finally, a uniform suspension of GO nanosheets was obtained by adding water to the resulting precipitate and 12 h of sonicating. The resulting suspensions were uniformly drop casted on a LightScribe DVD disk and then dried under the air at an ambient temperature. The GO coated DVD disk was placed in a LightScribe DVD drive with a wavelength of 780 nm. Raman result confirm that the laser irradiation properly reduce GO to graphene sheets. The structure of graphene arrays were studied by SEM images the results indicated that the laser based reduction was a useful method to production graphene layers. The CV curves of pristine rGO at various scan rates showed that the ultimate product has the power of storing energy in a supercapacitor level. Finally, the long-term charge-discharge stability of the LSG has been plotted which indicates that specific capacitance has decreased very slightly from its primary capacitance of ~ 10 F cm-3 and its cyclic stability is favorable over 1000 cycles.
Keywords:Graphene oxide; laser scribed graphene (LSG); scanning electron microscope (SEM); Cyclic voltammetry (CV); galvanostatic charge/discharge (CC)
Supercapacitors are a category of electrochemical energy storage devices that to some extent have advantages of both batteries and capacitors. Nowadays, an increasing use of portable electronic devices can be seen in industrial and experimental applications, medical equipments and even daily used cell phones and laptops in which the power storage and power durability are the must-have specifications of all these devices [1-4]. Miniaturized power storage devices are used in these applications, among which batteries and supercapacitors are the most important. Fast charge and discharge beside long cycle life and high power density are three properties to which give the supercapacitorsa great potential to complement or replace batteries [5,6].Carbon based materials play serious role in manufacturing supercapacitor electrodes [7,8]. Graphene, a 2D allotrope of carbon, possesses unique electrical and mechanical properties such as outstanding electrical conductivity, very high theoretical surface area of 2630 m2/g, promising flexibility and tensile strength of 130 GPa. Therefore, graphene nano-flakes are availed to be suitably applicable in supercapacitor and other energy storage devices [9-11].Graphene has unique properties such as nanometer size, high hardness and mechanical strength, high electrical and thermal conductivity, flexibility and magnetism. Due to this, many uses of this material, including its use in nanoelectronics, solar cells, energy storage devices such as batteries and supercapacitors [1]. In these structures, carbon atoms do not use one of their capacities. This empty capacity, which is in fact an additional electron, can be linked off the plate with other atoms. This free or suspended capacity can be linked to functional groups or other radical atoms in the environment [2]. Because of its special atomic composition in graphene, this material has a high conductivity and is very suitable for use in circuits and electronic devices. Graphene is a very diamond-like toughness, and has been widely used for it. Reduction of graphite oxide to graphene with laser irradiation were reported by El-Kady at science then other try to optimize its efficiency, other research groups try to reduce GO by electrophoretic methods or magnetron sputtering processes, all of them tried to support its reduction energy by different routes [12-14]. Due to its elastic properties and high pressure bearings, it is anticipated that there will be a lot of change in the industry. In the laser text method (LSG), graphene activates a superconductor with a high capacity for energy storage. We use the usual laser technique to make graphene screens on a disc surface, and then connect these sheets to the electrochemical capacitors (or supercapacitors). These supercapacitors can store a lot of energy in the same size as a typical battery, but can be discharged about 100 to 1,000 times faster. These capacitors are fully flexible and solid. This makes them ideal for energy storage systems for flexible and portable electronics [3,4]. Graphene is synthesized in a laser text method using a standard disc drive head, which is usually used as an optical device for engraving labels and images on disks. It can be seen easily when the golden graphite oxide turns black into graphene. This graphene-coated plastic is then used to make various devices with slices [5].
In electrochemical capacitors, graphene screens have a high surface area (more than 1 500 m2/g), which helps to increase their energy storage capacity and reach high electrical conductivity (over 1,700 siemens/m). They are also mechanically flexible and can be bent thousands of times without damaging their electrical properties [6,7].
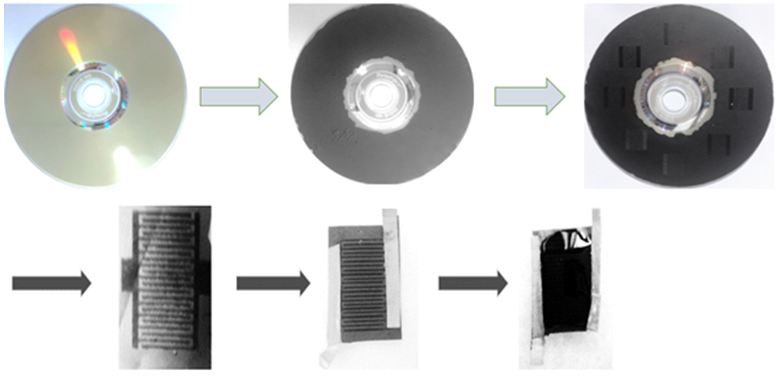
In this research, laser writing techniques for graphene synthesis on the surface of a disk and the production of graphene supercapacitors have been considered. For this, first, in the Hamers method, graphite was converted into an acidic environment consisting of sodium nitrate, potassium permanganate and sulfuric acid to graphene oxide. Centrifugal and ultrasonic devices were used to homogenize the graphene oxide solution at 3,000 rpm for another 45 min. The homogeneous graphene oxide solution was applied to the surface of 5 DVD discs and the set was dried at ambient temperature. To rebuild graphene oxide and convert it into graphene from a suitable laser, special supercapacitor modeling was used. The laser was performed by applying energy equal to the frequency of the graphene and oxygen bond escalation, breaking the connection and reducing the action and reaching graphene. The optimal laser wavelength was then determined to reduce the graphene oxide. The laser scribing of graphene was performed by LightScribe DVD drive with a wavelength of 780nm and a spot size of 20μm. In this study, the graphene synthesis and special supercapacitor modeling were performed in one step, which is the largest good method for laser graphene writing.
In the laser method, the graphite is oxidized and in a strong acidic environment, they separate the graphene oxide (GO) layers. This oxidation process produces a large number of oxygenated groups (such as carboxyl, epoxide and hydroxyl groups) at the surface of graphene. The presence of these polar factor groups, in some cases surface-ionized, greatly enhances the ability of graphene oxide, and as a result, it is easily spread in water and polar organic solvents. The unfavorable fact about graphene oxide is that the presence of functional groups at the surface removes graphene oxide from its unique properties. Graphene oxide is an electrically non-conductive structure whose layered structure is curved by the presence of a carbon-carbon bond [8].
One of the advantages of this method is the availability of suitable raw materials and other commonly used methods in Iran. The goal is to achieve a faster product and increase efficiency. However, controlling the test conditions is important in terms of temperature and the gases emitted. The laser techniques in the production of the supercapacitor include the steps of preparing graphene oxide solution, graphene synthesis and the construction of a supercooled capacitor. Graphene composite and molybdenum disulfide have been investigated to improve the performance of supercapacitor.
The graphene solution is prepared using sodium nitrate, potassium permanganate, sulfuric acid, oxygenated water, graphite and deionized water.
In a laser scratch technique, 2 g of graphite powder, 1 g of sodium nitrate and 46 milliliters of sulfuric acid are mixed and gently stirred to form a graphite oxide solution. Then the combined temperature of the ice bath is reduced to 10 °C, 6 g of potassium permanganate slowly is added slowly to the solution, so that the solution temperature does not rise above 20 °C (the reaction of permanganate with a thermosetting solution). The solution is then kept stable for 30 minutes at 35 °C. The color of the solution was initially grainy brownish, which, after 30 minutes, turned green in slime.
Then 92 ml of deionised water is slowly added to the solution. This step took place underneath the hood because when the deionized water was added, a violet steam emerged from the solution. Also, with the addition of deionized water, the solution temperature reached 100 °C and its color turns brown. After 15 minutes, the temperature is reduced to 65 °C and the color of the solution becomes darker. 340 ml of hydrogen peroxide was added to the solution, which became yellowish-brownish. Figure 1 shows an overview of the prepared solution [9].
The graphene oxide solution that exists contains acids, ions, etc., as shown in Figure 2, part A. To produce pure graphene oxide, dissolved in a fusion centrifuge, it is placed inside the 15th falcon under 3000 rpm for 5 minutes [10]. Because graphene oxide is heavier, it deposits, and oxides and the like are added, the acids are discharged, and then some deionised water is added by flickering the depleted water into the deionised water and dissolved graphene oxide plates, which in Figure 2 is shown in Part B. This diluted solution cannot be poured onto the disk. For this reason, the graphene oxide solution is placed in an ultrasonic device for 12 hours. Ultrasonic air bubbles cause the bubbles to penetrate the graphite oxide plates, and, with gradual enlargement and then bursting, the graphene oxide plates are separated and the homogeneity and concentration of the solution dissolved, in Figure 2c it has been shown.
Because the former diluent solution was heterogeneous and was not able to be applied to the disk, a concentrated solution of graphene oxide after ultrasonics can be dispensed onto the disk.
Finally, the solution was filtered using a suitable filter. At specified intervals, the filtered precipitate was washed with deionized water and hydrochloric acid (HCl) to prevent the presence of acid in the sedimentation of the residue. The dough was dried at a temperature of 65 °C for 24 hours, which eventually dipped the gray color according to Figure 3 [11].
This method of making graphene is more compatible with large-scale production. In this method, the graphite is oxidized, and in a strong acid environment, they separate the graphene oxide layers.
This oxidation process produces a large number of oxygenated groups (such as carboxyl, epoxide and hydroxyl groups) at the surface of graphene. The presence of these polar factor groups, in some cases surface-ionized, greatly enhances the ability of graphene oxide, and as a result, it can be easily spread in water and organic solvents. The unfavorable fact about graphene oxide is that the presence of functional groups at the surface will discard graphene oxide from its unique properties [15].
After preparing a solution of graphene oxide by laser method, using a high-performance laser method, and for making graphene-based flexible electrochemical capacitors for the synthesis of graphene and producing the desired component in one stage, which is the best method for laser scratching [16].
The laser will break through the energy of the resonance frequency of the graphene and oxygen bond, and thus the action will be reduced to reach graphene. In the laser scratch method, the most important issue is the use of laser to build a super capacitor, which requires the application of a special supercoaxial pattern. The most important method of laser scratching, graphene synthesis and component fabrication is in one step using laser programming, as shown in Figure 4 [17].
Making a Super Capacitor the laser device will engrave the special super-capacitor pattern on the surface as shown in the illustration below. The most important part of this lithography process, without the need for a mask, will be a great achievement for the first time in Iran. Graphene oxide is reduced to graphene by using a standard disc drive head, which is usually used to transparently engrave labels and images on disks. This process can be monitored easily when brown golden graphite oxide turns into black graphene.
Although electro-chemical capacitors (ECs) known as super-capacitors or super-capacitors have faster charging and discharging than batteries, they have limitations due to low energy density and low speed. In the laser scratch a standard laser optical drive was used to reduce the laser [18].
The film was resistant, showed high electrical conductivity (1738 sims per meter) and a good surface area (1520 m2/g), so it could be directly used as ordinary electrochemical capacitors without the need for a binder And flow collector. The equipment made with these electrodes shows very high specific density values in different electrolytes, so that for electrochemical capacitors, including high-density capacities and significant cyclic stability.
These electrochemical capacitors, with excellent electrochemical characteristics, are high under mechanical stresses, so they exhibit high power and electronic elasticity. Electrochemical batteries and capacitors have the potential to counterbalance the power and density of energy. Batteries store energy through electrochemical reactions and can display high energy density. While they store electrochemical charge capacitors in binary electrochemical layers [19].This EC shows a low charge / discharge ratio, which is a prelude to their use in high power applications. Recently, the EC equipment of curved graphite, graphite activated, and dissolved graphite has shown increased performance in terms of energy density. In particular, graphene electrodes produce a robust mechanical strength with a large thickness (approximately 10 μm or higher) and a high volumetric ratio in a binder process in high power EC and high energy density [20].
Here is a strategy for generating a graphene base EC based on a simple solid-state view that avoids the accumulation of graphene screens. The process is schematically shown in Figure 5. At first, a thin film of graphene oxide dispersed in water was cast into a droplet on a flexible substrate. Radiation and Radiation of the film with an infrared laser inside an optical drive the commercially available disk, written with light, reduces the graphene oxide for laser-graphene (LSG), as it is by changing the color of Brown to Black was shown [21].
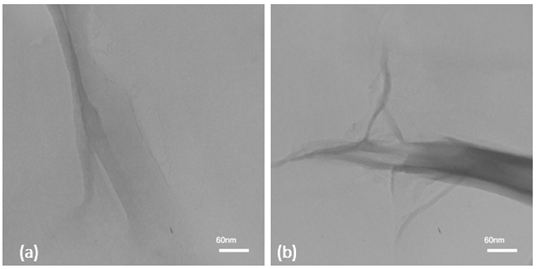
Analysis of transmitted electron microscopy in the preparation of a graphene oxide solution. In order to verify the quality of the graphene oxide solution, the output of the electron microscopy test is used to assure the formation of graphene oxide plates. In Figure 6, the formation of graphene oxide plates is observed. For this purpose, 5 ml of a solution of graphene oxide was poured into the sample electron microscope. And after drying the solution in the test environment.
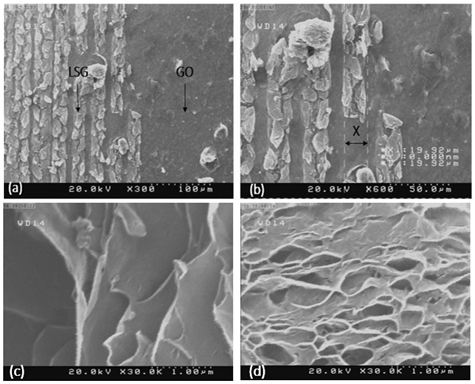
Scanning Electron Microscopy Analysis in Graphene Synthesis After graphene synthesis, the LSG graphene plates created alongside the graphene oxide phase were tested by scanning electron microscopy to form grapheme plates alongside the graphene oxide phase. Figure 7 provides the best view of the laser scratch method (a) and (b) the basis of the work by scratching the surface of graphene oxide with laser and synthesizing graphene with the phase change rate and generating graphene as an overview of the diameter of the laser beam It is found that it is about 92.9 μm (c) and (d) represents laser-synthesized graphene.
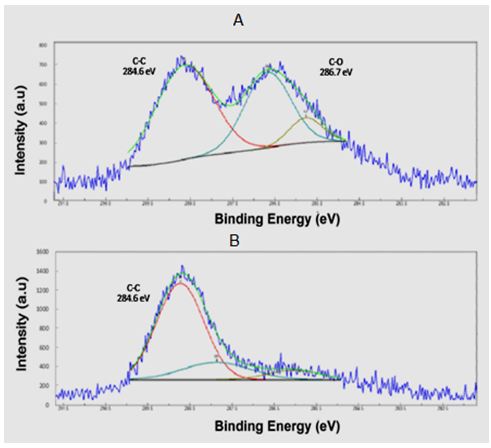
X-ray photoelectron emission spectroscopy analysis in graphene synthesis X-ray photoelectron spectroscopy is used to check the links between graphene and graphene oxide (Figure 8). X-ray photoelectron spectroscopy is used to check existing bonds before and after laser application. Before applying the laser, the carbon-oxygen bonds of graphene solution Carbon - carbon is evident, while after applying the laser and synthesizing graphene carbon-carbon bonds are present in the composition. It causes noticeable decrement in the ratio of oxygen to carbon indicating the reduction of GO to rGO.
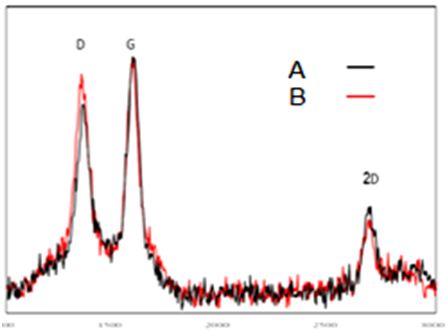
Raman test is to check the quality of the LSG graphene prepared by studying the G and D peak and their ratio, which represents the properties of the synthesized material. Raman spectroscopy is a kind of interaction between light and matter in which light is dispersed non-elastic. Figure 9 shows the Raman test to check the quality of the LSG graphene, as it is noticed. There are two basic bands in the Raman spectrum. Courses D and G are the wavelengths of the Raman spectrum of 1327 cm-1 and 11578 cm-1. G band is due to graphite mode (sp2 band) and band D due to diamond fashion (sp3 band). However, the height of the 2D peak in this analysis indicates the quality of the synthesized graphene. Raman and XPS results confirm that the laser irradiation properly reduce GO to graphene sheets. As the sharp peak of 2D band in Raman spectrum presents, fabricated sheets have few layers.
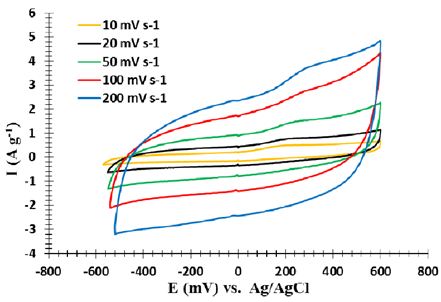
The electrochemical properties of a standard three-electrode cell are investigated through cyclic voltammetric testing, galvanostatic charge and discharge, and impedance spectroscopy. Voltammetric tests are carried out at room temperature using platinum electrodes as auxiliary acetate and an Ag/AgCl electrode as the reference electrode. A half-molar KCl solution has been used for experiments. Impedance spectroscopy is carried out at an open circuit potential through a sinusoidal signal with a range of 5 mV in the frequency range of 0.1 to 10,000 Hz. The supercapacitor performance was analyzed through two cyclic voltammetric tests and galvanostatic charge/discharge tests. The superconducting supercomputer exhibits a roughly quadrilateral crossover model with an elastic capacitor behavior. Figure 10 illustrates the relatively high gradient curve during charging and discharging (the initial part of the diagram and the initial part of the return graph), indicating a high charge and discharge rate. Also, during the cycle, the cycles of returning to these graphs are not a specific courier, which means that there is no oxidation and regeneration during the process.
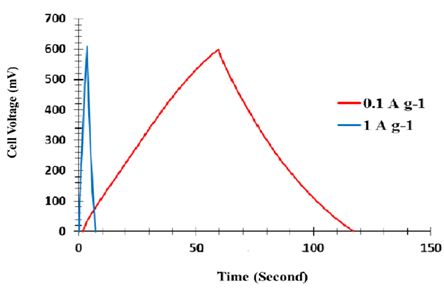
Although no metal flow collectors, binders and electrostatic extras have been used, they are commercial electrochemical supercapacitors. In addition, these supercapa ones are strong enough to charge and discharge a wide range of scanning rates, and still have an almost perfect quadrant for CV. Figure 11 shows the result of galvanostatic charge and discharge. As shown in this figure, the charging and discharging sections of the graph have almost mirror symmetry relative to each other. This indicates that the charge and discharge are reversible in the supercapacitors tested.
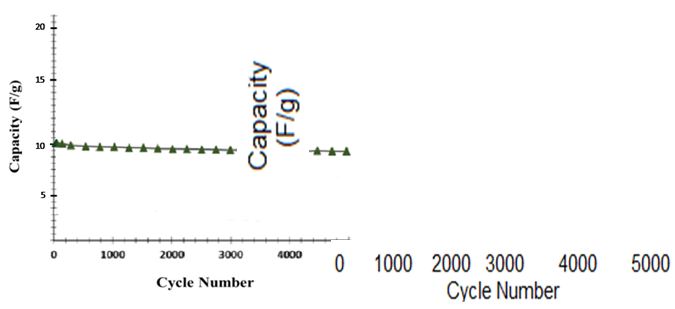
In Figure 12, the dependence of the capacitive capacitance of the super capacitor on the discharge current is shown. This figure shows that the capacitive capacitance decreases with a slight slope as the charge and discharge current increases. So the result we find that all of the electrodes exhibit excellent charge and discharge properties even in high discharge streams. So they can be a good option for super-capacitive industrial super-capacitors. These electrochemical supercapacitors can be effectively charged / discharged. Meanwhile, LSG-EC holds 96.5% of its initial capacity after 1000 cycles.
Laser technology in the LSG method and non-mask lithography is the most important distinction of the laser scratch method with similar universal methods. The applied laser will be scratched and shown in (Figure 2B), which shows the difference between the LSG method and the general methods of using laser for the synthesis of graphene by lithography by sweeping the entire surface. The most important stage of programming is the laser to apply a specific wavelength to graphe oxide, in fact, the laser will break energy by as much as the resonance frequency of the graphene bond and the oxygen bond and by applying a special pattern of supercapacitor for the synthesis of graphene and the production of supercapacitors In one step, the best laser scratch method is. The laser reduced the coincidence of the sheet of sheets of graphene oxide and simultaneously produced a LSG network. This structure prevented the agglomeration of graphene plates, which was a major obstacle to achieving the full potential of the superconductor, with a special high surface area of more than 1500 square meters per gram. Graphene synthesized in the laboratory scratch experiment with a finite scale, but the greatest benefit of this method is the possibility of generating a large collection of electronic devices, such as supercapacitors, with faster and more efficient performance than similar methods. Raman test is to check the quality of LSG graphene prepared by studying the G and D peak and their ratio representing the properties of the synthesized material. However, the height of the 2D peak in this analysis indicates the quality of the synthesized graphene. The super capacitor's galvanostattic experiments have a relatively large slope over the charging and discharging time, indicating a high charge and discharge rate. Also, during the cycle, the cycles of returning to these graphs are not a specific courier, which means that there is no oxidation and regeneration during the process. Electrochemical supercapacitors in the laser method can be effectively charged / discharged and hold 97% of the initial capacity after 1000 cycles.