Fungicidal Activities of Cu (II) Soaps Derived From Various Oils Treated at High Temperature for Biomedical Use
Biologically potent compounds are one of the most important classes of materials for the upcoming generations. Increasing number of microbial infectious diseases and resistant pathogens create a demand and urgency to develop novel, potent, safe and improved variety of antimicrobial agents. This initiates a task for current chemistry to synthesize compounds that show promising activity as therapeutic agents with lower toxicity. Therefore, a substantial research is needed for their discovery and improvement. The antifungal activities of copper soaps derived from various oils treated at high temperatures have been evaluated by testing against Alternaria alternata and Aspergillus niger at different concentrations by agar plate technique and it is found that these Cu (II) soaps are very effective on these. The fungi toxicity results indicate that the strain of fungal species are susceptible towards these soaps and suggests that with the increase in concentration of copper soap it may increase further.
Keywords: Copper surfactants; Anti-fungal studies; Edible oils
Most of the antimicrobial agents are obviously organic compounds, which behave as good chelating agent. Their pathogenic activity is enhanced on complexing with various transition and toxic metals [1,2]. The biological effect of these derivatives depends on the nature and structure of ligands and their metal complexes and also on the presence of particular element. Many copper thiosemicarbazone complexes are found to have significant antitubercular, fungicidal and antitumor activities [3-5]. The use of copper linoleate as heavy duty wood preservative and many other biological activities of copper metal containing surfactants also have been studied [6,7]. Formulated proprietary brands of cuprous oxide are extensively employed as fungicides and seed dressings. Copper oxychloride has a number of applications, by far the most important being as an agricultural fungicide for which purpose it is extensively employed in formulated form as dusts, wettable powders and pastes [8].
Small quantities of copper soaps such as copper stearate, copper oleate and copper abietate (from resins), are employed mainly for rot-proofing textiles, ropes, etc. The main applications of copper sulfate are in the production of proprietary wood preservatives and agricultural fungicides [9,10]. In our earlier communication antifungal activities of neem, soyabean, sesame, groundnut and mustard oils, and their complexes with N,S donar ligands like Urea, thiourea, Benzothiazole have been reported. Mathur, et al . studied the biological evaluation and DNA binding cleavage studies of copper soaps and their complexes [11-19].
All the above studies suggest that the copper metal play a vital role in fungicidal activities. Various edible oils are widely used, easily available and economic. These facts led us to synthesis copper soaps of some oils and their treated oils remaining after frying, to study their micellar characteristics and role of copper metal in fungicidal activities for exploring their applications and other possible uses in various industries and agriculture. For this purpose, we have chosen some fungicidal activities of the synthesized copper soaps against easily available fungi Alternaria alternata and Aspergillus niger.
These oils are easily available in the Indian and chosen for the investigation. Their compositions are recorded in Table 1. All the chemicals used were of LR/AR grade. Three samples for each oil have been prepared as fresh (untreated), treated oil at high temperature for 15 minutes and for 60 minutes.
About 500 g oil was taken in an iron pan. The oil was heated to the highest temperature and 5 g potato chips were deep fried while maintaining the frying temperature between180-200 °C. The oil was heated for 15 min. in the open air and sunlight in iron pan at 180-200 °C. The volume of the oil was not replenished to the original volume after frying operations. The frying experiment with both oil samples at 180-200 °C for 60 minutes was conducted in similar manner.
Cu (II) soaps were prepared by refluxing the oils with 2N KOH solution and alcohol for about 3 h (Direct Metathesis). Copper soap so obtained was then washed with warm water and 10% alcohol at 50 °C and recrystallized using hot benzene Molecular weight of copper soap was determined from saponification value of the oil sample prepared. The formation of copper soap was confirmed by using elemental analysis, UV, IR techniques.
Fungicidal activities: The general laboratory techniques followed in the course of this investigation are as suggested by Booth and Hawks worth [20,21].
Glasswares used in our study were of pyrex brand. The glasswares viz. test tubes, conical flasks, pipettes (micro and macro), glass rods and petridishes were thoroughly washed after rinsing with chromic acid each time before sampling. The petriplates and other glassware were then sterilized in hot air oven at 160 °C for 24 hours before use.
The cultured medium used for the growth of the organism in the present study was P.D.A. (Potato dextrose Agar). The following media was used in the present study
Potatoes-200g
Dextrose-20g
Agar-20g
Distill water-1000ml
200g of potatoes were cleaned, cut into pieces and boiled in about 1000 ml of tap water for 2 h. Then, the contents were strained using muslin cloth. To this extract, 20 g of dextrose, 20 g of agar were added and made up to 1000 ml in graduated flask, before sterilizing the medium.
All the copper soaps derived from untreated and treated oils were tested for antifungal activity. The calculated amount of the soap was weighted in a standard flask and the solutions containing different concentration (1000 and 10000 ppm) of soaps in benzene were prepared
In the present study, following two test organism are used:
A. Alternaria alternata
B. Aspergillus niger
The above organisms were isolated from their natural habitat and then purified, characterized and identified [22] (Figure 1a,1b).
Fungicidal Testing
1 ml of the soap solution was aseptically transferred into sterile petriplates. Into these plates, 20 ml of P.D.A. was poured and was mixed with soap solution by rotating the petriplates in clockwise and anticlockwise direction 3-4 times and was allowed to solidify.
After the solidification of the above medium, single hypha / spore of test organism were aseptically transferred in the centre of the petriplates. The plates were incubated at 30 °C for 3 days. After the period of incubation, the plates were observed for the growth of fungus in different concentration of the soap solution used in the present study. The data were statistically analysed according to the following formula:
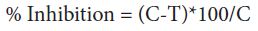
Where C = diameter of fungal colony in control plates after 3 days T = diameter of fungal colony in test plate after 3 days
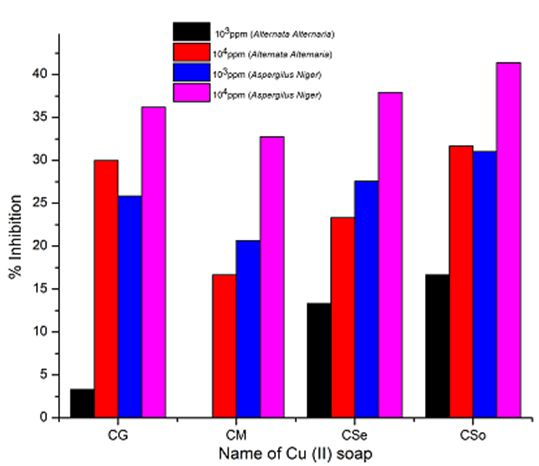
Copper soaps derived from untreated and treated oils have been screened for their antifungicidal activity against Alternaria-alternata and Aspergillus-niger at 1000 ppm and 10000 ppm by Agar-plate technique. Copper soaps showed moderate activities against both the fungi.
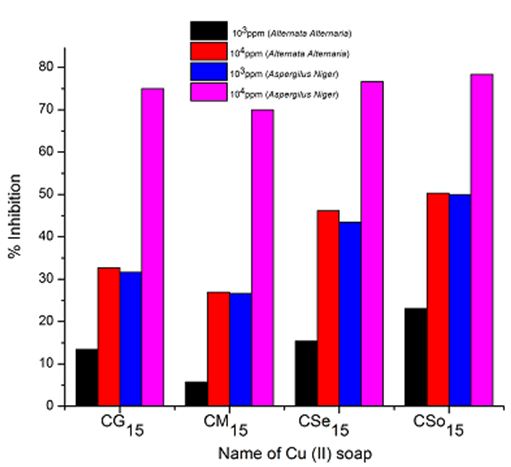
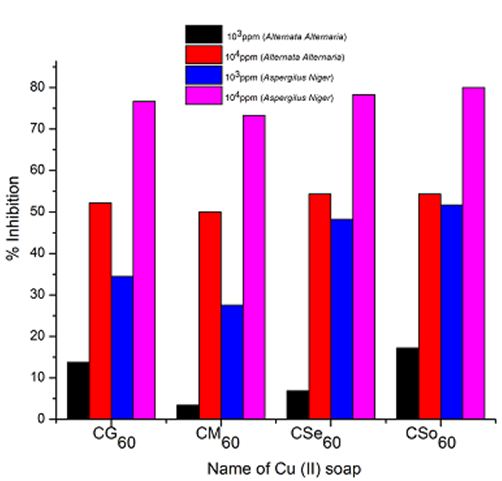
A perusal of Figure 2, 3 and 4reveals that all the copper soaps have significant fungitoxicity at 10000 ppm but their toxicity decreases markedly on dilution (at 1000 ppm). From the figs, it is apparent that their efficiency increases with their concentration. Thus it is evident that concentration plays a vital role in increasing the degree of inhibition. Fungicidal screening data revealed that at lower concentration the inhibition of growth is less as compared to higher concentration
From comparison of the results for both the fungi, it is found that all copper soaps are more potent (more toxic) against Aspergillus niger than against Alternaria- alternata i.e. inhibition of growth is higher for Aspergillus niger than inhibition of growth for Alternaria alternata. The results are concise with our earlier reported work on soaps with complexes derived from saturated fatty acids, edible and non-edible oils [23-26].
A perusal of the figs shows that CM is the least fungitoxic (% inhibition lowest) whereas CSo is the most toxic against both fungi. The activity (toxicity) of copper soaps derived from untreated oils are found to increase in the order For copper soaps derived from treated oils for 15 minutes, the results are same as copper soaps derived from untreated oils. CM15 is the least active and CSo15 is the most active against both fungi. The order of activity of copper soaps derived from treated oils for 15 minutes is as follows [27]
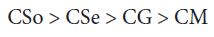
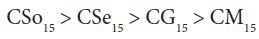
For copper soaps derived from treated oils for 60 minutes, CM60 is the least active but difference in activity of these copper soaps is not so high and order of activity is as follows:
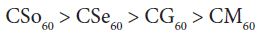
From comparison of copper soaps derived from untreated and treated sample of oil, it is found that fungitoxicity increases with the increase of time of heating for oils i.e.
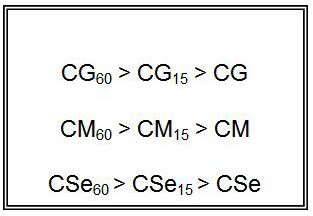
All the tests were performed in triplicate the standard deviation has been measured by the conventional measure of repeatability and the average was taken as final reading. The results of ANOVA for the antifungal activities for all soaps has been done, predicted R2 are in reasonable agreement and closer to1.0. This confirms that the experimental data are well satisfactory [28]. The descriptive statics results of Cu (II) soaps shown that SE and SD are 0.5 % which confirms satisfactory results in triplet The result is statistically significant, by the standards of the study, due to p < F [29,30].
It has been observed that the antifungal activity increases with the increase in the concentration of the solution. From comparison of copper soaps derived from untreated and treated sample of oil, it is found that fungitoxicity increases with the increase of time of heating for oils.
The authors would like to thank Principal and Head of Department of Chemistry, S.D. Govt. College, BEAWAR and S.P.C. Govt. College, Ajmer- Raj. India for providing Laboratory Facilities.