Genotoxicity Assessment of the Hospital Wastewaters in Tunisia
Hospitals release considerable amounts of chemicals in their wastewaters. These effluents can be hazardous to the environmental and the human health. In this study we evaluated the in vivo and in vitro genotoxicities of three wastewaters collected from three regions in Tunisia (Gafsa, Mahdia and Sfax cities). DNA fragmentation assay was conducted on the liver of Swiss albinomale rat after a daily treatment (during 28 days) with different doses of the hospital wastewaters. Our results showed that all of the hospital wastewater exhibited high qualitative and quantitative changes in the integrity of the genomic DNA extracted from the hepatic tissues. Genotoxicity increases proportionally to the concentration of the tested effluent, but the effluents collected from Sfax hospital revealed higher genotoxicity than the two others. This result could be explained by the presence in this effluent of some toxic micropolluants. However, the output of the three tested hospital wastewaters induced a non-significant genotoxic effect on the Salmonella typhimurium TA104 prokaryotic VITOTOX assay in the presence or the absence of microsomal fraction S9. In fact, the gentox/cytox ratio did not exceed the threshold 1.5.
The present study showed that the hospital wastewaters in Tunisia exhibited potentially detrimental toxicological risks, and thus, the hospital effluents treatment requires special attention.
Keywords:Biomonitoring; Hospital wastewater; Genotoxicity; Water pollution
Nowadays, pharmaceutical products become indispensable to the contemporary lifestyle. However, recent studies showed the emerging problem of pharmaceutical-based pollution of river environments, including drinking water sources and lakes, which have begun receiving significant interest worldwide. Because pharmaceuticals are designed to perform specific physiological functions in targeted regions of the human body, there is an increasing concern regarding their toxic effects, even at low concentrations, on the human health. Hospitals constitute the principal spot where pharmaceuticals are consistently employed to treat diseases [1]. Many studies have been conducted on the hospital effluents which showed that these contain large amounts of pathogenic organisms, micro pollutants, and dangerous and persistent substances such as pharmaceuticals, radionuclides, solvents, disinfectants, and metals [2-4]. More recently, pharmaceuticals raised scientific and public concerns regarding their potential impact on the environment and human health. In fact, the hospital effluent can generate a large amount of pollutants with teratogenic, carcinogenic, and mutagenic hazards [5-7]. Health risks of associated with the long-term toxicity of hospital wastewaters are not well known. Indeed, in most countries, hospital effluents are discharged directly into public sewers and are introduced into the municipal wastewater networks where they are treated with other effluents. There are currently no limits on allowing discharges, and hospitals are not obliged to process to specific treatments of their wastewaters [8]. Also, we previously demonstrated the toxic effects of some pharmaceuticals, such as antibiotics [9-11]. In fact, the effluent containing antibiotics has revealed genotoxic and cytotoxic effects and can induce many damages to the tissue of marine species.
Thus, the aims of this study were to characterize the wastewaters obtained from three different Tunisian hospitals (Mahdia, Gafsa and Sfax) and to evaluate their genotoxic potentials. In this context (i) Genotoxicity and cytotoxicity were evaluated using Salmonella typhimurium TA104 prokaryotic Vitotox assay (ii) Genotoxicity was also evaluated in vivo in the liver tissue of rats after daily treatment during 28 days.
The water samples were collected on March 2017 from three Hospital’s wastewaters localized in Mahdia City, Gafsa City and Sfax. Geographical coordinates are as follows:
* Hedi Chaker University Hospital Sfax: latitude 34.740725 |longitude 10.750304&
* Tahar Sfar University Hospital Mahdia: latitude 35.510075|longitude 11.032643
* Regional Hospital Gafsa: latitude 34.420007|longitude 8.796143
The sampling points were located on a main drain, receiving the highest amount of wastewater from the selected hospitals. These wastewaters contained discharges from the following departments: general medicine, general surgery, intensive care units, maternity, gynaecology, oncology, psychiatry, rheumatology, haematology, hepatic-gastroenterology, several radiology units, and laboratories. Wastewater samples collected were transported to the laboratory prior to determining the different physicochemical parameters and/or immediately stored at -20 °C until further analysis.
Experimental animals
Sixty adult male rats (weighing 180-200 g) were purchased from SIPHAT, Tunisia and their standard pellet diet was purchased from the Industrial Society of Rodents’ Diet (SNA, Sfax, Tunisia). The animals were maintained under constant temperature (22±1 °C) and humidity (50%), with diurnal lighting (12 h light/ 12 h dark). Rat were fed with a standard laboratory diet and given free access to tap water. All animals were treated according to the local Institute Ethical Committee Guidelines for the Care and Use.
Animal’s treatment
After two weeks of adaptation, animals were randomly separated into ten groups with six rats in each group: Group (1) control group receiving distilled water; Groups (2), (3) and (4) rats receiving 25, 50 and 100% concentrations respectively of a pharmaceutical effluent collected from the hospital of Gafsa; Groups (5), (6) and (7) rat receiving 25, 50 and 100% concentrations respectively of a pharmaceutical effluent collected from the hospital of Sfax; F) rats receiving 25, 50 and 100% concentrations respectively of a pharmaceutical effluent collected from the hospital of Mahdia. Effluents were administrated orally to all the groups by gavage for 28 consecutive days.
Biological samples collection
At the end of the experimental period (28 days), the rats were sacrificed by cervical decapitation. Liver tissues were excised immediately from the rats and stored in a liquid nitrogen container to avoid protein degradation and were used for molecular studies.
DNA fragmentation assay
Qualitative damage to genomic DNA was estimated by agarose gel electrophoresis, as previously described by Kanno, et al. [12]. DNA was extracted in duplicate from the liver with equal volumes of phenol-chloroform-isoamyl alcohol (25:24:1), and precipitated with twice the volume of ethanol. DNA samples (3 μg of DNA/lane) were electrophoresed on 1.4% agarose gel containing ethidium bromide (final concentration, 0.16 μg/ml), and the fragmentation was visualized under UV light.
The test was carried out with Salmonella typhimurium TA104 that are genetically modified, and it shows DNA damage by emitting a readily-detectable signal of bioluminescence after exposure to mutagenic factors [13]. The first day, overnight pre-cultures were conducted for both Genox and Cytox strains in a rich medium. Pre-cultures contained 100 μl of each strain (cultures were stored at 80 °C), 625 μl of tetracycline (0.8 mg ml-1) and 312.5 ml of ampicillin (8 mg ml-1) and the two Erlenmeyer’s flasks were placed in a water bath (300 rpm, 36 °C) for 16 h. The second day, the two Erlenmeyer’s flasks were placed on ice for 15 min. In Erlenmeyer’s flasks containing 20 ml of mineral medium, 160 μl of previously cultured bacteria were added and stirred during 1 h in an appropriate water bath (300 rpm, 36 °C). These Genox and Cytox work-cultures are then ready for testing. A mixture of 2.125 ml of mineral medium, 350 μl of bacterial suspension and 1 ml of the post-mitochondrial supernatant fraction S9 was prepared and transferred to a black 96-well microplate with 100 μl from each sample (diluted at ½ and ¼ in distillate water). When the S9 is not used, we added 1 ml of mineral medium. Positive controls were used, 4-NQO (0.4 mg ml-1) tested without S9 and B (a) P (0.8 mg ml-1) tested with S9. A micro plate luminometer was used to measure genotoxicity and cytotoxicity; the light was analyzed every5 min over a 4h time span. All the calculations were automatically performed and based on measurements taken between 60 and 240 min of incubation. The tested compounds were added to the bacteria, in the presence and absence of the S9, which was obtained from the livers of aroclor-treated rats. The signal to noise ratio (S/N) represents the calculation of the exposed bacteria light production divided by unexposed bacteria light production for each measurement and for each strain separately. Previous experiments have demonstrated that genotoxicity always takes place when the maximum S/N (genox)/maximum S/N (cytox) is greater than 1.5 and cytotoxicity is assumed when S/N in Genox/Cytoxdecreases far below 0.8 [13-15].
The monitoring of water contamination for potentially genotoxic compounds represents a major concern for human health. This study offers data to consider such a concern using samples from three Hospital wastewaters and different toxicity bioassays.
All the wastewater samples showed a direct genotoxic effect (Genox/Cytox ratio >1.6) in dose dependent manner when tested in the absence of S9mix (Figure 1). However, any genotoxicity was observed when hospital wastewaters were tested in the presence of S9 mix. In fact the Genox/Cytox ratio does not exceed 1.5 (Figure 2).
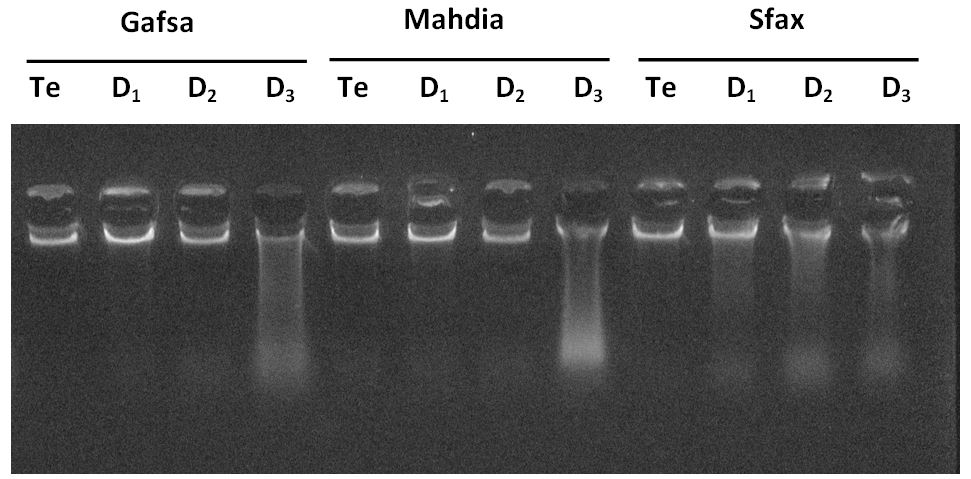
The gel electrophoresis DNA isolated from liver of control samples (lane 1, 5 and 9), 25% and 50% concentrations of the pharmaceutical effluent treated animals collected from Gafsa and Mahdia, respectively (lane 2, 3, 6 and 7), presented an intact band (Figure 3). But, in these regions, a clear nuclear DNA fragmentation, a hallmark of apoptosis, characterized by a mixed smearing and laddering of the DNA were observed in 100% concentrations of the pharmaceutical effluent treated group (lane 4 and 8). In Sfax region, all the three doses of pharmaceutical effluent were able to cause DNA fragmentation in the liver of treated rats when compared to the control group (lane 10, 11, and 12).
The occurrence of toxic substances in different hospital effluents has been reported by many studies [16,17]. The detection and the quantification of these residues in the wastewaters are considered to be of particular concern because of their genotoxic potential which causes adverse environmental effects even at very low doses [18,19]. Previously studies, we focused, on the detection of heavy metals and antibiotics in hospital wastewaters from the three different regions in Tunisia (Mahdia, Gafsa and Sfax) [3].
It is well known that the pharmaceutical residues found in hospital effluents reflect prescription trends and patients consumption [20]. It has revealed the presence of high concentrated effluents such as Enrofloxacin, Marbofloxacin, Oxytetracycline, pipemidic acid sulfamethoxazole, sulfonamides, acetaminophen, mefenamic acid, Atenolol, carbamazepine and Caffeineand personal care products such as benaophenone-3 and benzotrizole were the most frequently detected in the three hospital effluents (results not shown). The tested hospital effluents are considered as the main source of the release toxic metals (e.g. Cr, Co, Ni, Cu, Zn, Cd, Pb and Hg) into the environment [3]. It is well known that hospital effluents, industrial effluents and domestic wastewaters can contain pharmaceutical compounds, drugs, chemicals and pesticides, detergents and disinfectants could also be detected in the wastewaters [21]. The genotoxic activity of such mixtures was already demonstrated in many previous studies [22-25].
All the three effluents showed a genotoxic activity, so we can deduce the presence of different genotoxic compounds formation.
The addition of S9 mix addition does not boost the response and the hospital wastewaters having a low genotoxic response. Our results were in agreement with other study which showed that the putative genotoxins in surface waters and municipal wastewaters are primarily direct-acting, i.e., S9 addition does not advance the response and the municipal wastewaters having a low genotoxic response. However, we were in disagreement with Kittinger, et al., where a toxic signal could only be found after addition of S9 mix.
Indeed, the conventional water quality indices and chemical analysis give little information on possible aftermaths and the numerous formed substances that can threaten human health [21,23]. So, further histological assays should be performed in order to detect changes in cellular organics such as the presence of micronuclei after exposure to different concentrations of wastewaters. Thus, chemical agents can induce micronuclei through spindle disturbance or chromosome breaks [27].
Apoptosis plays an important role in the development and maintenance of homeostasis in most of the multicellular organisms [28]. Fragmented DNA test is the most widely utilized test for the genotoxic and mutagenic assessment of xenobiotic due to its technical simplicity and less time consumption [29]. The results of present study revealed that sub-acute exposure to different concentrations of the pharmaceutical effluent collected from Gafsa, Sfax and Mahdia was associated with an increase of DNA fragmentation in rat liver, a hallmark of apoptosis. Many authors have suggested a link between genotoxicity and pharmaceutical effluent poisoning [30-34]. This type of cell death can be triggered by the activation of pro-inflammatory cytokines that ultimately culminates in the activation of caspase family of proteases [35], or by activation of free radicals, either through pharmacodynamics, auto-oxidation, or enzyme-catalyzed oxidation of electrophilic molecule of the pharmaceutical effluent [32]. Indeed, reactive oxygen species, endogenously generated on exposure to the effluent, could induce lipid peroxidation in the tissues by reacting with the lipid content of the cell membrane, thereby causing breakage of the DNA chain by oxidating the base component of the membrane. In addition, the free radicals generated by effluent could also have reacted with those protein-enzymes involved in the DNA repair mechanism, the alteration of repair enzyme activity resulting in increased frequency in DNA damage [36-38].
In conclusion, The results obtained in this study clearly indicates that the effluent emanating from the regional hospital in the cities of Mahdia, Gafsa and Sfax are directly discharged into the municipal wastewater networks and produce cytotoxic and genotoxic damage, despite of the low concentrations of metals and other contaminants. Much as, it is necessary to acquire additional information about the prevalence and levels of contaminant agents produced in hospitals, in order to design environmental control programs for the pre-treatment of wastewater before its release into municipal sewage systems.

.JPG)
.JPG)
.JPG)
.JPG)
.JPG)
.JPG)
.JPG)
.JPG)