Giant Hepatocellular Adenoma in a Young Male: Report of an Extremely Rare Case
Hepatocellular Adenoma (HCA) is a rare primary benign hepatic tumour, seen in young and middle- aged women and associated with the use of Oral Contraceptives (OCP). Though generally asymptomatic, large hepatic adenomas are complicated by rupture and spontaneous bleeding with increased risk in tumours larger than 7cm. The presentation is varied and these tumours are extremely rare in males affecting mainly females with the highest prevalence in reproductive age and located in the right hepatic lobe with greater frequency. This case was unusual as it occurred in a male patient and in the left lobe of liver which is extremely rare. We report such a rare case in a 30-year-old male with no predisposing factor who presented with upper abdominal pain and was diagnosed to have a giant HCA, inflammatory subtype (HA-1) measuring 7.5 x 8 x 2.5 cm by histopathological confirmation of resection specimen.
Keywords: Hepatocellular adenoma; Hepatic resection; Rupture; Malignant transformation
Hepatocellular adenoma (HCA) also known as hepatic adenoma (HA) or liver cell adenoma is a rare primary benign neoplasm of hepatocellular origin comprising 2% of all liver neoplasms with an incidence of 3/1000000 per year in European and North American countries [1]. It commonly affects young women with a long-term history of oral contraceptive (OCP) use and is increasingly being detected due to widespread use of radio imaging modalities [2-4]. HCA may be also found in association with conditions like diabetes mellitus, pregnancy, Fanconi anemia, Hurler disease, Familial Adenomatous Polyposis (FAP) and tyrosinemia [5-8]. Rarely, HCA has been associated with abusive use of anabolic-androgenic steroids, seen mainly among body builders and weight trainers [9-11]. Though generally asymptomatic, large hepatic adenomas are complicated by rupture and spontaneous bleeding with increased risk in tumours larger than 7cm [12].
A 30-year-old male with no known co-morbidities presented to our hospital for management of abdominal pain in the right upper quadrant for the past 3 months. The pain was mild to moderate in intensity, non-radiating with no aggravating or relieving factors. There was no associated history of fever, jaundice, vomiting, hematemesis, melena or abdominal distention. Family history was non- contributory. There was no history of any long-term medication in the past. General examination revealed normal vitals. Pallor was present however no icterus, pedal edema, generalized lymphadenopathy or cyanosis was present.
On per-abdominal examination, the abdomen was soft in consistency with no guarding or rigidity. Tenderness was present on deep palpation in the right hypochondrium and liver was enlarged with a span of 17 cm. A single soft to firm lump measuring approximately 4 x 4 cm was palpable just below the xiphisternum, extending transversely from the midline up to 4 cm. The liver could not be palpated separately from the mass. There was no free fluid and bowel sounds were present. His liver function tests were normal and alpha fetoprotein was within normal limits.
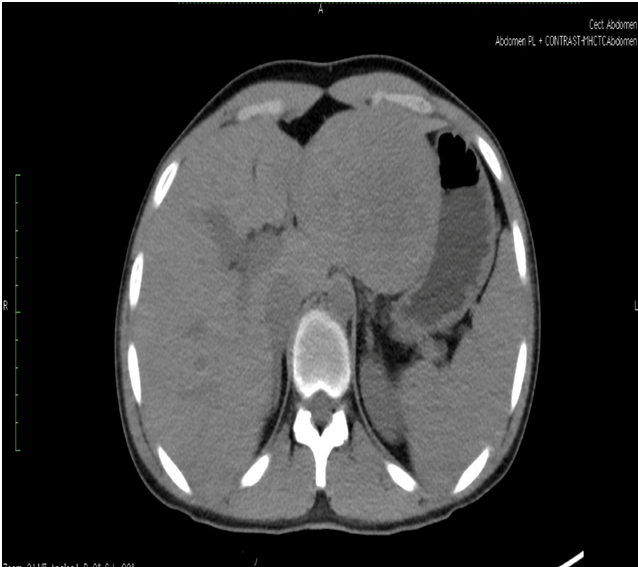
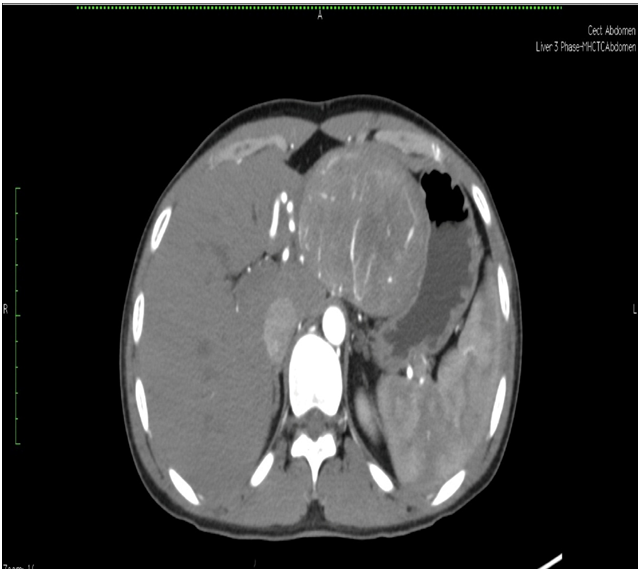
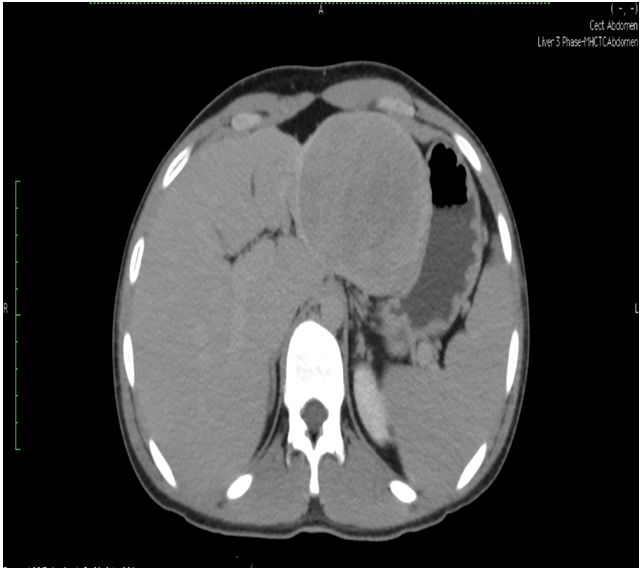
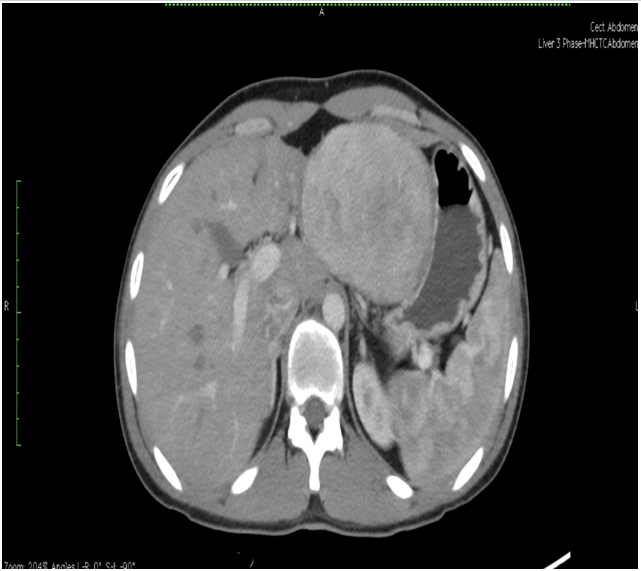
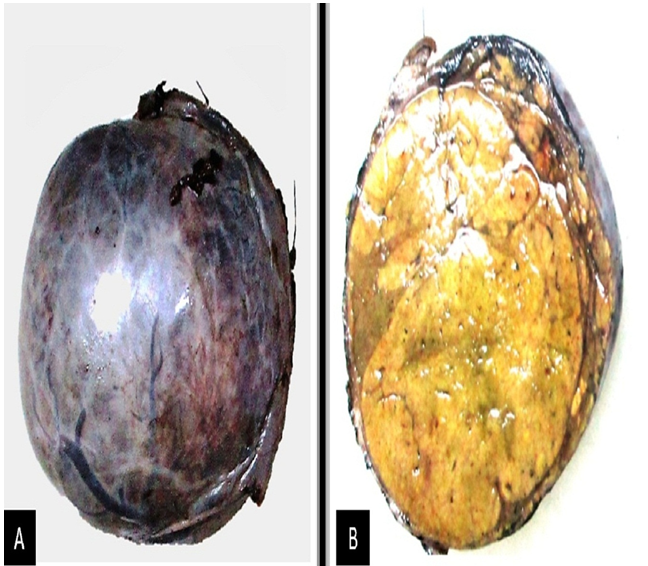
Triple phase CT scan of abdomen was done which was suggestive of a homogenously enhancing capsulated benign hepatic mass lesion in the left lobe indenting the right wall of body of stomach (Figure 1,2,Figure 3 and Figure 4). Resection of the mass was done under General Anaesthesia (GA) and intra-operative findings revealed presence of a Space Occupying Lesion (SOL) in the left lobe of liver. Grossly the specimen measured 11 x 12 x 3 cm. Cut section showed a well-circumscribed tumour measuring 7.5 x 8 x 2.5 cm. The tumour was yellowish in colour and soft in consistency with few areas of haemorrhage. No necrosis was seen (Figure 5).
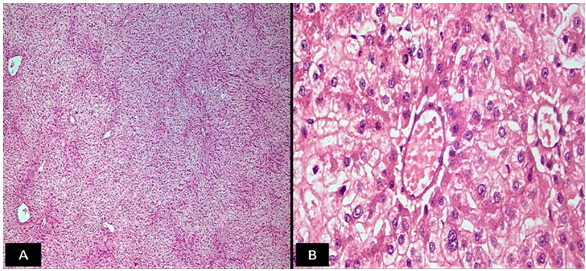
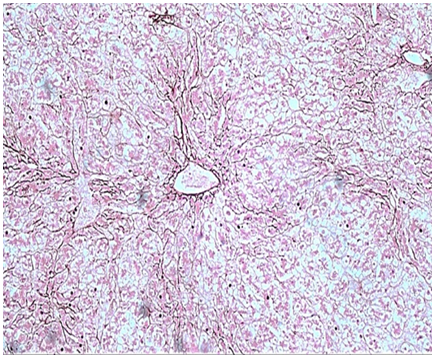
The routine Haematoxylin and Eosin (H & E) stained sections examined from the mass showed a well circumscribed tumour composed of hepatocytes arranged in cords and trabeculae which were 1-2 cell thick plate separated by sinusoids. Pseudo-portal tracts were prominent along with presence of mild inflammation comprising of lymphocytes, histiocytes, neutrophils and plasma cells. Portal tracts were absent. Kupffer cells were reduced to absent. The cells lacked cytological atypia with minimal nuclear pleomorphism (Figure 6). No mitosis was noted and few areas of haemorrhage were seen. No necrosis noted. Normal hepatic parenchyma was seen at the periphery. Reticulin stain revealed presence of intact hepatic architecture with minimal plate thickening (Figure 7). Based on these features a diagnosis of HCA inflammatory subtype (HA-I) was rendered. Postoperative period was uneventful. There was no recurrence or metastasis during one year of follow up.
HA is a benign tumour that was rare before the introduction of OCPs in 1960. A possible association between use of OCPs and the development of hepatic adenoma was first described by Baum in 1973 [13]. These tumours are clinically associated with OCP use in women and occasionally anabolic corticosteroid use in men [13]. Our case was peculiar as it occurred in a young male without any usual predisposing factors and the diagnosis in this unusual clinical setting was challenging. The diagnosis of HCA is important as it can rupture, leading to life threatening haemorrhage. They also have a small propensity to transform into hepatocellular carcinoma (HCC) with an overall risk of 4.2% [14-16]. The clinical presentation varies from majority of patients being asymptomatic to those with symptoms, usually right upper quadrant pain secondary to HCA bleeding, which can present as internal haemorrhage with necrotic changes mostly observed in adenomas > 4 cm or there can be spontaneous rupture that causes subcapsular hematoma and possible hemoperitoneum [17]. Approximately 70% -80% of HCA are solitary lesions usually located in the right lobe of liver but our case showed a left lobe lesion which itself is very unusual [18,19].
Clinical evaluation may reveal a palpable intra-abdominal mass or enlarged liver in less than 30% of cases [18]. Radiological imaging with Ultrasound (USG), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are used to determine the diagnosis, but it is difficult to accurately distinguish between HCA and other lesions, such as Focal Nodular Hyperplasia (FNH). In such cases, a liver biopsy is necessary to establish a diagnosis [17-20]. On macroscopic examination, HCAs are unencapsulated and solitary tumours varying from 1- 20cm with areas of haemorrhage and necrosis [19]. Microscopically HCA appear as monotonous sheets of hepatocytes lacking biliary structures, fibrosis or dysplasia. There are 4 subtypes of HCA; (1) HA-H Subtype shows steatosis and an absence of inflammation or atypia; (2) HA-B subtype shows pseudoacinar formation with mild atypia, There is no steatosis or inflammation; (3) HA-I subtype has pseudoportal tracts that without bile ducts or veins and show inflammation. Inflammatory infiltrate is predominantly mononuclear as was seen in this c-20ase. HA-I is the most common variant (35–50 % of HCAs). These have an increased risk of bleeding and are associated with small risk of malignant transformation; (4) HA-U Subtype which shows adenoma-like features but does not have any distinctive features [12,19]. Novel tissue analysis techniques such as Q-Bend 10, erbB2-immunostaining and fluorescence in-situ hybridization (FISH) may help overcome the dilemma of accurate tissue diagnosis [21].
The role of elective surgical resection for HCA is still controversial and mainly depends upon the risk of complications, the uncertain diagnosis and the presence of symptoms related to tumor size and site, particularly with regard to the risk of rupture and resulting haemorrhage [22]. Malignant transformation is rare, but there have been a few cases reported [14]. In one series of 39 cases of unresected liver adenoma, 5 cases subsequently developed HCC [23]. Sometimes HA is difficult to distinguish from a well differentiated HCC. Therefore surgery should be indicated when clinical diagnosis of hepatocellular carcinoma can’t be ruled out or malignant transformation is suspected. In our case the clinical findings, radio-imaging and histopathological examination helped in arriving at the definitive diagnosis. Alternate therapeutic modalities such as selective arterial embolization (SAE) and radio-frequency ablation (RFA) may be useful in cases not amenable to surgery [24]. Liver transplantation may be reserved for unresectable patients or those with malignant transformation, symptomatic with recurrent or ruptured adenoma [25,26].
We hereby report an unusual and sporadic case of a large HCA in a male who had no predisposing factors which was successfully resected. The diagnosis of a HCA can be suggested by a combination of imaging modalities, but owing to the non-specificity of imaging findings, tissue diagnosis is a necessity. Histological confirmation can also be difficult as low grade HCCs can look similar to HCAs. Ultimately, large HCAs should be surgically removed owing to potential haemorrhage and the small possibility of malignant transformation. Documenting the occurrence of HCA in an unusual clinical setting will give an insight into the natural history of this very rare tumour.
We extend our gratitude to SAJ Cancer Science publishing group for providing complete waiver of processing and publication fees. We thank the patient for allowing us to publish the case report and use the images taken during his stay in hospital. The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and each author believes that the manuscript represents honest work.