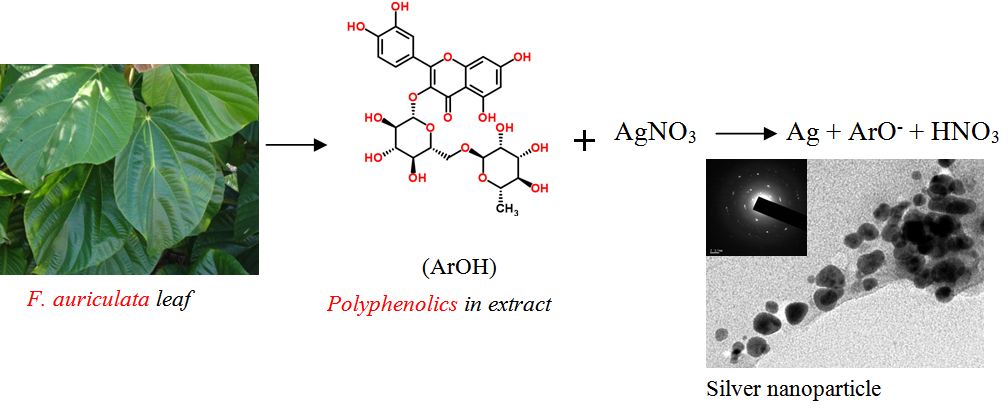
Green Synthesis of Silver Nanoparticles Using Phyto-Constituents of Ficus Auriculata Lour. Leaf Extract: Mechanistic Approach
Green synthesis of silver nanoparticles from silver nitrate using Ficus auriculata leaf decoction as a reducing agent has been elucidated with possible mechanism of reaction. Preliminary phytochemical screening of the extract revealed the presence phenolics, carbohydrates, flavonoids and saponins. HPTLC fingerprinting and further UPLC-Q-TOF mass spectroscopy analysis confirmed the presence of the flavonoid glycoside, rutin in the extract. The extract showed good antioxidant property in DPPH model. SEM and TEM analysis showed that the nanoparticles formed were of different morphologies with an average size of 19-21 nm. FT-IR spectral analysis showed the role of hydroxyl groups in the phytoconstituents as reducing agent in synthesis. The synthesized silver nanoparticles showed good antimicrobial potential when analysed against Photobacterium leiognathi, a very sensitive and accurate biosensor, by luminometry. Synthesized silver nanoparticles showed good morphological, dimensional and pharmacological properties.
Graphical Abstract
The antioxidant polyphenolics such as rutin presents in the F. auriculata leaf extract acted as reducing agent in the green synthesize of silver nanoparticle. Possible mechanism of action and bioactivity are also elucidated.
Keywords: Antioxidant Property; Ficus auriculata; Green Synthesis; Silver Nanoparticles
Silver nanoparticles (AgNPs) exhibit improved properties such as plasmon resonance characteristics depending upon their size and morphologies [1]. Silver nanoparticles become the focus of intensive research owing to their wide range of applications in catalysis, optics, electronics, antimicrobials, cosmetics, biosensor fabrication and biomedical applications [2]. There are several reports on the antioxidant, cytotoxic properties of AgNPs and its potential applications as a drug carrier for the treatment of cancer [3]. Green synthesis of nanoparticles is now evolving as an important branch of nanotechnology and there are considerable interest in applying the principles of green chemistry and sustainability to industrial organic synthesis. Green synthesis of metal nanoparticles using plant extracts has several advantages over chemical, physical, and microbial syntheses, because it is devoid of the use of any hazardous chemicals, high-energy requirements, toxic by-products or the elaborated process of culturing and maintenance of microbes [4].
Biosynthesis of nanoparticles is a kind of bottom up approach, where the main mechanism of action is redox reaction. The need for biosynthesis of nanoparticles arises from the fact that the physical and chemical processes became costly and the use of hazardous chemicals in various steps [5]. Phytochemical mediated metal nanoparticle syntheses are effective, economical and environmental friendly. Plants are considered as biosynthetic laboratories of wide spectrum of phytochemicals such as phenolics, alkaloids and flavonoids. These phytochemicals are expected to self assemble and cap the metal nanoparticles formed in their presence and thereby induces some shape control during metal ion reduction [6]. Metallic nanoparticles obtained by biosynthesis employing plant extracts have been reviewed and reported by different authors [7,8].
Ficus auriculata, Lour., a type of fig tree seen all over Asia, belonging to the Moraceae family, has been traditionally used for treating wounds, diarrhoea, dysentery, mumps, cholera and vomiting [9]. In the present study, we have investigated the phytochemical composition of F. auriculata leaf extract, its antioxidant potential and its use in the biosynthesis of silver nanoparticles as reducing agent. The antioxidant property, its correlation with total phenolic content and possible class of phytocostituents responsible for the reduction is also elucidated. Toxicity of the synthesized silver nanoparticles were analysed by luminometry using photo luminescent bacteria as biosensor.
Silver nitrate (AgNO3) was obtained from Merck, India. Deionized water was used throughout the reactions. All glass wares were washed with dilute nitric acid (0.5 M) then distilled water and dried in hot air oven.
Plant material was collected from the university campus and air dried in shade. The leaf powder (10 g) was extracted with 200 ml of double distilled water by boiling for 30 min. The extract was filtered, centrifuged at 10000 rpm for 10 min. and the supernatant was stored at 4 ºC.
A small portion of the dried extract dissolved in methanol were analyzed for the presence of secondary metabolites such as tannins, flavonoids, steroids, glycosides, cardiac glycosides and saponins by characteristic colour reactions [10].
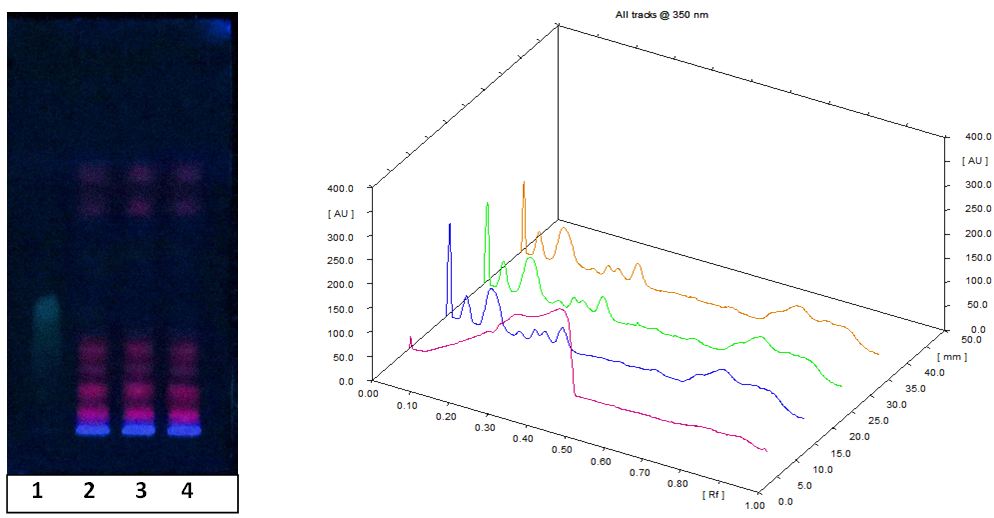
The leaf extract and the reference sample, quercetin (0.1 mg ml-1 in methanol) were spotted as bands with Hamilton syringe on a pre-coated silica gel plates 60F 254 (5 cm × 10 cm, Merck) using CAMAG linomat V applicator. Samples were spotted with a band width of 6 mm. The plates were developed in the solvent system (Chloroform: Ethyl acetate, 3:2 v/v) in CAMAG glass twin trough chamber previously saturated with the solvent system for 20 min. Solvent front was marked as 8 cm. TLC plates were air dried and scanning was performed on Camag TLC Scanner 4 in fluorescence mode at 360 nm with Hg lamp. The sample application control and data analysis were performed with winCATS software.
UPLC analysis was performed on reversed phase column (BEH C18 column, 50 mm × 2.1 mm × 1.7 μm) on Acquity H class (Waters) Ultra Performance Liquid Chromatography. A binary system of H2O + 0.1% formic acid (solvent A) and acetonitrile (solvent B) gradient was used for elution for 10 min. In these conditions, analytes were eluted from the column in decreasing order of polarity. Several linear gradients were assayed. Peak detection was done at 254 nm. MS analysis was performed on Xevo G2 (Waters) Quadrapole – Time-of-Flight (Q-TOF) mass spectrometer. For MS analysis, the same optimised solvent system was used as mobile phase. The mass range for scanning was between m/z 50–1000. Electrospray operating conditions were optimized for analysis (dry temperature 350 °C, nebulizer pressure 50 psi, capillary voltage 2.5 KV, cone voltage 35V and source temperature 135 °C).
Phenolic estimation was performed by Folin - Ciocalteu’s phenol reagent method [10]. 0.4 ml aliquot of the plant extract (1000 μg/ml) was transferred into a test tube containing 0.8 ml of the 10% Folin-Ciocalteu phenol reagent (Sigma). 1.6 ml of the 10% sodium carbonate solution was added after 3 min. The contents were mixed well and left to stand at room temperature for 1 h. Absorbance were recorded at 750 nm (Perkin Elmer UV/Vis Spectrometer- Lamda 650). Gallic acid (HIMEDIA) was used as reference standard. The experiment was triplicated; the observations were reported as mean ± SD and expressed as μg equivalent of gallic acid/1000 μg sample.
The HPTLC chromatographic plate after development was sprayed with 0.1 mM DPPH solution in methanol (Merck, India; 99.8% purity). The positive activity was detected by the pale yellow spots on a reddish purple background due to the decolourization of DPPH by the antioxidant. Quercetin dihydrate was used as reference standard.
The free radical scavenging activity was measured in terms of hydrogen donating or radical scavenging ability, using the stable radical, DPPH [11]. The scavenging reaction between (DPPH•) and an antioxidant (H-A) can be written as:
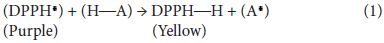
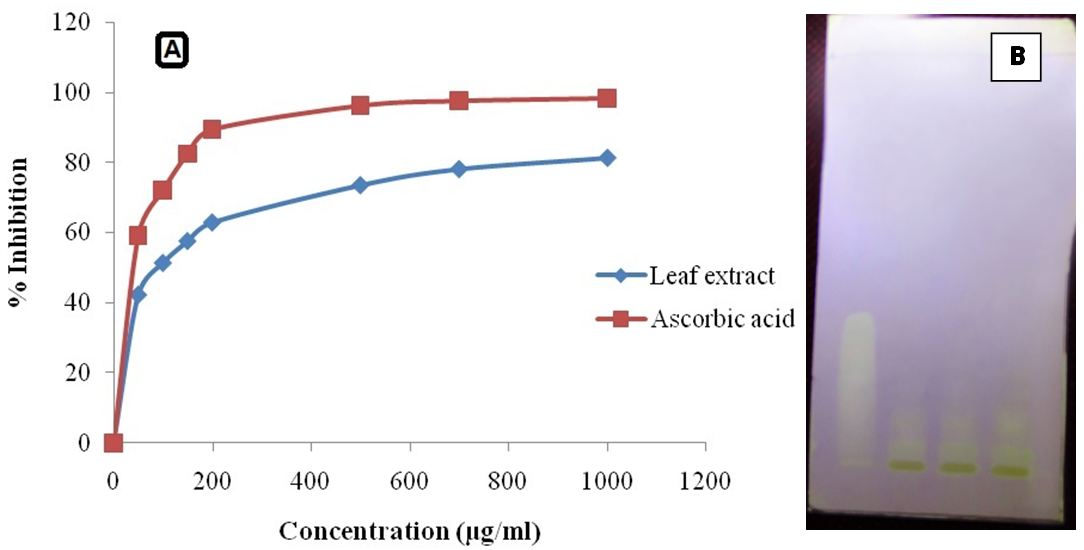
The degree of discoloration indicates the scavenging potential of the antioxidant compounds in the extracts in terms of hydrogen donating ability. 1.0 ml of methanolic solution of 2, 2-Diphenyl-1-picrylhydrazyl solution (0.1 mM) was added to 3.0 ml of control (without the test compound, but with an equivalent amount of methanol), while test solutions and standard ascorbic acid (HIMEDIA) at different concentrations (50-1000 μg/ml). The absorbance was measured at 517 nm after 30 min. of incubation. Samples were tested in triplicate. Percentage inhibition was calculated by comparing the absorbance values of control and test samples.
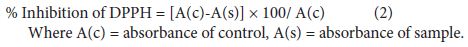
45 ml of Silver nitrate solution (0.001M) and 5 ml of leaf extract were mixed and constantly stirred with a magnetic stirrer maintained at 45 °C. Synthesis of silver nanoparticles was monitored periodically by UV-Visible spectral scanning in the range 300-600 nm. A UV-Vis spectrograph of the colloidal solution of silver nanoparticles was recorded as a function of time on Perkin Elmer UV/Vis Spectrometer (Lamda 650).
Purification of the synthesized nanoparticles was optimized by recording the absorption spectrum of supernatant after each centrifugation at different rpm (5000, 10000, 15000 and 20000) till the disappearance of specific peak. Finally the nanoparticles were washed with Millipore purified water and harvested by centrifugation and then dried at low temperature.
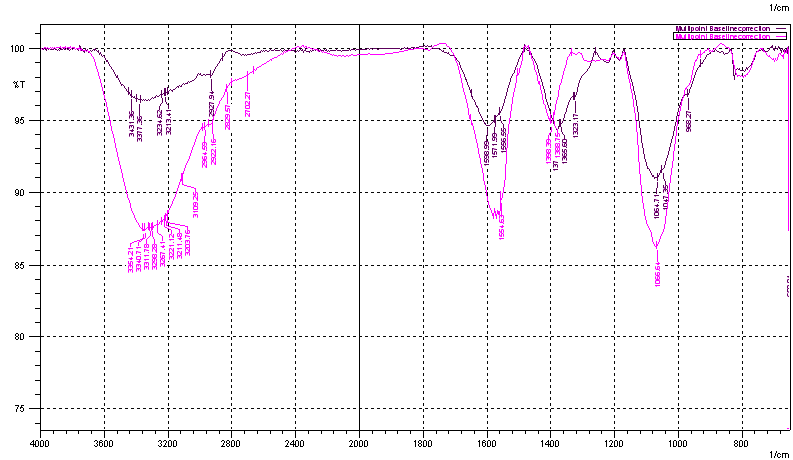
FT-IR analysis of the dried extract before and after nano-synthesis was done by using Shimadzu IR Prestige-21 spectrophotometer. The absorption spectra were recorded from 650 to 4000 cm-1 in ATR mode using ZnSe crystal. 20 scans were averaged per spectrum.
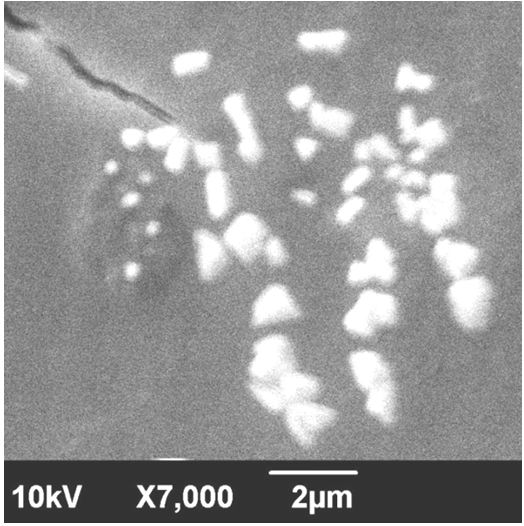
The sample was suspended in double distilled water and deposited by simple drop coating on carbon tape held by the SEM sample holder for analyses. The solvent (water) was allowed to evaporate and dried. It was then sputter coated with platinum and analyzed in a high-resolution scanning electron microscope (JEOL JSM-6390).
The sample was suspended in double distilled water and deposited by simple drop coating on carbon tape held by the SEM sample holder for analyses. The solvent (water) was allowed to evaporate and dried. It was then sputter coated with platinum and analyzed in a high-resolution scanning electron microscope (JEOL JSM-6390).
Toxicity of silver nanoparticles was studied by using TOX-SCREEN Test Kit (CheckLight). The test kit contains light producing (luminous) bacteria, Photobacterium leiognathi, as very sensitive and accurate biosensors. The assay was based on the principle, toxic compounds in sample causes luminous bacteria to change the level of emitted light by inhibition. The test was performed as per the protocol given in the manual. 10 μl of biosensor, 400 μl of sample (1000 μg/ml) and 1590 ml of Pro-Metal assay buffer were mixed well, serially diluted and incubated for 15 min. Luminance was then measured on luminometer (Junior LB 9509, Berthold Technologies).
Qualitative phytochemical analysis of F. auriculata leaf extract showed the presence of saponins, glycosides, phenolics, flavonoids, tannins, phytosterols, terpenoids, and amino acids (Table 1). Ahlam, et al., (2011) reported the presence of compounds such as betulinic acid, lupeol, stigmasterol, bergapten, scopoletin, β-sitosterol-3-O-β-D-glucopyranoside, myricetin, and quercetin-3-O- β-D-glucopyranoside in F. auriculata leaf, which supports the current observation of complex nature of the extract [12].
Estimation of total phenolic contents in the leaf extract using the Folin-Ciocalteu’s reagent is found to be 62 ± 0.813 μg of gallic acid equivalent/mg of the sample. High solubility of phenolic compounds in polar solvents provides high concentration of these compounds in the water extract [13,14].
HPTLC analysis showed about nine phytochemical constituents in the extract and most of them showed orange red fluorescence at 366 nm (Figure 1). Fluorescence is the basic characteristics of polyphenols, flavonoids such as anthocyanins (Figure 2). On spraying the developed chromatogram with 0.1 mM DPPH solution showed that phyto-constituents of the plant decoction possess antioxidant property similar to reference standard, quercetin dehydrate, with white to pale yellow bands on pink background due to decolourization of DPPH by the antioxidant constituents (Figure 2B).
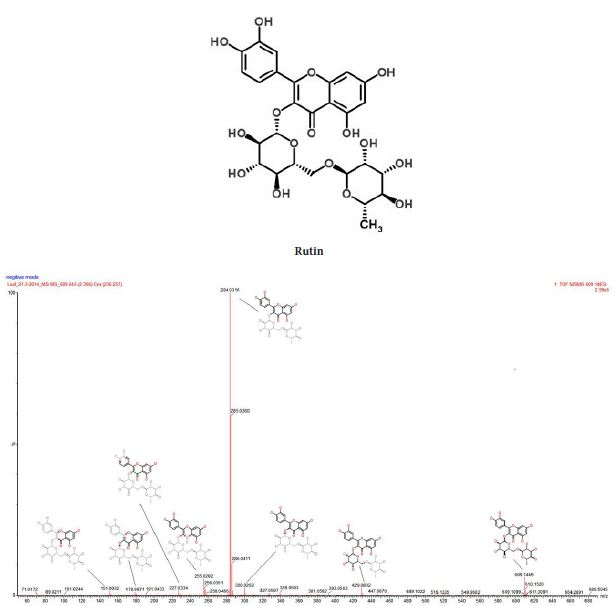
The UPLC chromatogram showed the presence of about thirteen components in the sample characterized by different retention time. Mass spectra of major peaks have been evaluated by taking the MS/MS spectra and fragmentation pattern. Presence of the potent antioxidant rutin has been confirmed by comparing the fragmentation pattern of the parent compound with the help of ChemSpider and associated software of Xevo G2 (Waters) Quadrapole – Time-of-Flight (Q-TOF) mass spectrometer (Figure 3). Rutin (quercetin-3-rhamnosyl glucoside), is a low molecular weight polyphenolic compound that is widely distributed in vegetables and fruits. Reducing capacity by electron donation, antioxidant potential and other pharmacological properties of rutin are reported in detail by Yang, et al. (2008) [15].
The extract showed good antioxidant potential with concentration as a measure of DPPH radical scavenging activity (Figure 2A). Polyphenolic constituents such as flavonoids and tannins may contribute to its reducing power or antioxidant potential [11]. Phenols are very important plant constituents because of their free radical scavenging ability, due to their hydroxyl groups. In vascular plants, more than 4000 phenolic and polyphenolic antioxidant compounds such as phenolic acids, tannins, coumarins, anthraquinones, flavonoids, phenolic diterpens and anthocyanin have been identified [16]. They have the ability to scavenge free radicals, donate hydrogen atoms or electrons. High correlation has been reported between antioxidant capacity and total phenolic content of plant extracts [17].
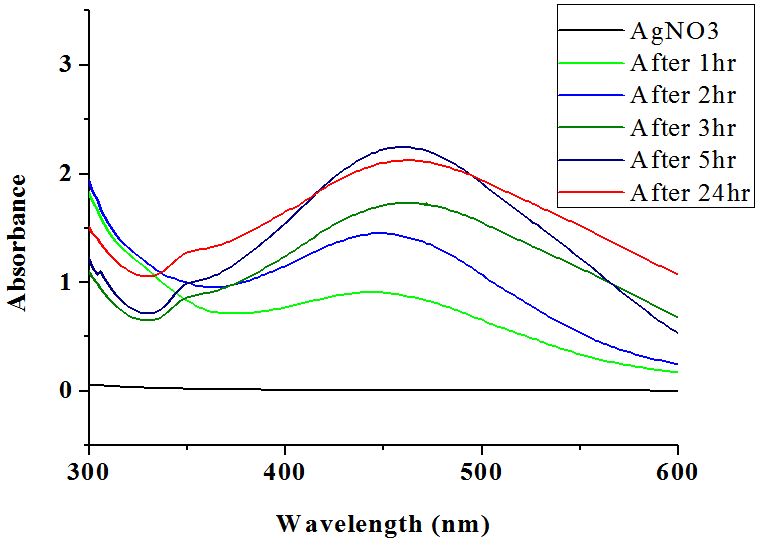
The appearance of yellow colour in the reaction mixtures which gradually changed to reddish brown tinge indicated the formation of silver nanoparticle formation [3]. Furthermore, the nanoparticle synthesis was assured by monitoring the absorption spectra of synthesized colloidal solutions against extract blanks. It showed a broad absorption band near the region of 451 nm and the intensity was found to increase with time (Figure 4). The distinctive colour of silver nanoparticle colloidal solution is due to the phenomenon known as plasmon absorbance. Incident light creates oscillations in conduction electrons on the surface of the nanoparticles. The collective oscillation of conduction electrons within metal nanoparticles, the surface plasmon resonance, enables scattering and absorption of light at a particular frequency, giving them the colour [17].
Silver nanoparticles were harvested by centrifugation of the colloidal nanoparticle solution at 15000 rpm or above for 10 min. The supernatant did not show any characteristic peak beyond this centrifugation speed and time. Further the precipitate was washed with double distilled water and centrifuged. A portion of the precipitate, redistributed in water, showed a broad peak at around 450 nm, indicated the stability of synthesized silver nanoparticles.
The FT-IR spectrum obtained for F. auriculata leaf extract displays a number of absorption peaks, reflecting its complex nature (Figure 5). Strong absorption peaks at 3263 to 3331 cm−1 result from stretching of the –NH band of amino groups or is indicative of bonded –OH hydroxyl group. The absorption peaks at about 2929.87 cm−1 could be assigned to stretching vibrations of -CH2 and -CH3 functional groups. The intense band at 1585 cm−1 could be assigned to the aromatic C=C bending. FT-IR study indicates that the hydroxyl (−OH), and amine (N-H) groups in F. auriculata leaf extract are mainly involved in reduction of Ag+ ions to Ag0 nanoparticles. Presence of rutin and other flavonoid glycoside confirmed by LC-Q-TOF mass spectrometric studies supports this observation. There was noticeable difference in the peak intensities of –OH and –NH functional groups, demonstrates the role of phenolics and amino acids in the reduction process. The FT-IR spectroscopic studies have also showed that the leaf extracts not only acts as reducing agent but as a stabilizer also, which prevents the agglomeration of AgNPs. The carbonyl group of amino acid residues has a strong binding ability with metal, suggesting the formation of a layer covering silver nanoparticles which act as a stabilizing agent to prevent agglomeration in the aqueous medium [4].
Water extract of F. auriculata leaves showed good antioxidant property when analyzed by DPPH assay. This antioxidant potential is mainly contributed by the phenolic phytochemicals such as flavonoids and reducing sugars or glycosides. LC-Q-TOF mass spectra analysis showed that this contains the flavonoid glycoside, 2,5-Dideoxy-1-O-{6-O-[(2S,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)tetrahydro-2-furanyl]-β-D-glucopyranosyl}-3-C-isopropylpentitol (rutine) and Hexyl 2-O-(6-deoxy-α-L-galactopyranosyl)-β-D-galactopyranoside. The antioxidant activity of these molecules is related to their molecular structure, more precisely to the presence and number of hydroxyl groups, to double bond conjugation and resonance effects [18]. Recently, a quantum–mechanical investigation has shown that the antioxidant action of flavonols is related to radicals showing a planar conformation that allows extended electronic delocalization between adjacent rings [19]. Wright, et al., (2001) has proposed two main mechanisms to explain the antioxidant potential of these molecules [20]. In the first mechanism, referred as H-atom transfer, the free radical removes a hydrogen atom from the antioxidant (ArOH) that itself becomes a radical:
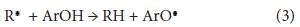
A higher stability of the radical ArO. corresponds to a better efficiency of the antioxidant ArOH. In the second mechanism (the one-electron transfer), the antioxidant can give an electron to the free radical becoming itself a radical cation:
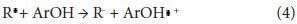
The stability of the radical cation generated by the electron transfer determines its activity. In this case, the lower the ionization potential, the easier is the electron abstraction. Thus polyphenolic compound with conjugated ring structures can show antioxidant property either by donating a proton or electron. This supports the observed correlation between phenolic concentration and antioxidant property.
The main reaction responsible for green synthesis or biogenesis of nanoparticle is the reduction of metal ions by various biomolecules such as proteins, sugars, etc. or the phytochemicals such as polyphenolic compounds. The reduction potential of these molecules determines the effectiveness of the reaction. In case of silver nanoparticle synthesis, AgNO3 will be reduced to the zero valent silver nanoparticles, Ag0. On reduction of AgNO3 the polyphenol will be converted to a phenoxide ion or phenoxyl radical. After the O-H bond is broken, the radical is able to rearrange itself to assume the most stable conformation. The probable reaction can be represented as:
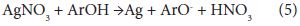
where the ArOH represents the polyphenolic compound. The antioxidant property of various polyphenolic compounds by proton and electron transfer mechanisms have been evaluated and the possible stabilization of oxidized radicals or ions of antioxidant molecules by resonance and other mechanisms are reported [21]. These phenolic compounds may bind over the silver clusters and facilitate easy reduction of ions over the surface to build up silver nanoparticle.
SEM images of synthesized silver nano particles showed different morphologies. The resulting nanoparticles showed spherical particles in the size range of 18-21 nm. SEM images also showed the presence of nanotriangles and other morphologies in the same suspension (Figure 6).
TEM analysis reveals that the Ag nanoparticles are predominantly spherical with an average size of 19-20 nm. Further, the capping ability of leaf extracts was also observed. The selected area electron diffraction (SAED) pattern of the silver NPs shows a ring-like diffraction pattern indicates that the particles are crystalline (Figure 7).
Silver nanoparticles showed much significant reduction in the luminance (RLU) of Photobacterium leiognathi compared to the negative blank solution, which is devoid of silver nanoparticles. Nanoparticle solution at a concentration of 200 μg/ml showed a luminescence of 63 RLU, while that of blank was 8927 RLU. Toxicity was found to be concentration and time of treatment dependent. Shrivastava, et al. have shown that silver nanoparticles may modulate the phosphotyrosine profile of putative bacterial peptides that could affect the cellular signalling and therefore inhibit the growth of bacteria [22]. The study performed by Hwang, et al., (2008), revealed that silver nanoparticles and the silver ions move into the cells and leads to the production of reactive oxygen species [23]. Based on the greater tendency of silver ions to strongly interact with thiol groups of vital enzymes and phosphorus containing bases, it is likely that further damages could be caused by interactions with compounds such as DNA by the silver nanoparticles inside the cells [24]. This interaction may prevent cell division and DNA replication, which may ultimately lead to cell death.
Green synthesis of nanoparticles is an eco-friendly approach possesses several advantages such as low toxicity, good yield, simplicity in synthesis and is economical. This study for the first time demonstrated the ability of F. auriculata leaf extract for the rapid synthesis of stable silver nanospheres and other morphologies. The leaf extract acted as both reducing and stabilising agents. The Ag NPs synthesised were found to have an absorption peak at 451 nm, characteristic to surface plasmon resonance property of silver nanoparticle. TEM micrographs of the resultant Ag NPs indicate the presence of spherical nanoparticles with an average diameter of 20 nm. Subsequent SEM studies showed that the synthesized nanoparticles consist of multiple morphologies. Phytochemical analysis and IR spectroscopy elucidated the mechanism of action of plant extract in the reduction of AgNO3. Phenolic hydroxyl and amino groups of the proteins, glycosides and other polyphenolic phytochemicals are the main contributors to the reduction as evidenced from the IR spectrum. HPTLC and LC-Q-TOF mass spectroscopic studies revealed the presence of wide spectrum of complex phytochemicals such as rutin in F. auriculata leaf decoction. Rutin is a polyphenolic flavonoid with potent antioxidant potential. The antimicrobial activity showed by the synthesized silver nanoparticles indicates that green synthesis is a potential route for metal nanoparticles synthesis for biomedical applications. In the medical scenario, biologically synthesized nanoparticles are found to be more effective due to their low toxicity. The antimicrobial activity of silver nanoparticle can be exploited for wound dressings, ointments, water treatment and other applications such as implant coatings. It may also find application in cancer treatment, drug delivery and even in enzyme immobilization.
Financial support from DST, New Delhi (under Purse scheme) and DST-Nanomission, Govt. of India is gratefully acknowledged.