Implant Augmentation for Trochanteric Fractures with an Innovative, Ready to Use Calcium-Phosphate-Cement
Background: It has become established practice to augment osteosyntheses with polymethylmethacrylate. However, material-specific problems remain unsolved. The aim of this study was the dynamic biomechanical testing of Calcium-Phospat-Cement for the augmentation of an intramedullary nail in a trochanteric fracture model as a biological alternative to polymethylmethacrylate.
Method: Osteosynthetic stabilization of a trochanteric fracture model was conducted on 8 pairs of human cadaver femurs with a nail. The implant was augmented randomly in one bone of each of the pairs. For augmentation with CPC, cannulated telescopic screws were prepared and the thread section was cemented onto them. The load was progressively increased (0.5 to max. 2.1 kN, at 4 Hz) under imaging guidance. The endpoint of the test was a cut-out or completion of the dynamic testing without a cut-out after 200,000 alternating loads.
Results: In the CPC augmented group, there were significantly fewer cut-outs (p<0.05). In the non-augmented group, there was a strongly significant correlation between BMD and the number of cycles until the endpoint (rho=0.67; p=0.03). This correlation did not exist in the augmented group. The stiffness at the start and after 10 k cycles was significantly higher in the augmented group (p=0.01 t start; p<0.01 after 10 k cycles). There was also a significant negative correlation between the stiffness measured at 10 k cycles and the cut-out rate (r=0.44; p<0.05). There was a not statistically significant trend in favor of the augmented group in the number of cycles until the endpoint (119.75k ±54.25 k cycles non-augmented vs. 162.25k ±54.92 k cycles augmented; p=0.11).
Conclusion: This biomechanical study showed biomechanical advantages after CPC-augmentation and an increase in the number of load cycles compared with the non-augmented control group. Additional studies using technically optimized cannulated telescopic screws to achieve better cement distribution and involving a biomechanical comparison of augmentation with PMMA and CPC are needed.
Keywords: Proximal Femur, Trochanteric Fractures, Cement Augmentation, Targon PF, Cutout, Calcium-Phosphate Cement
Due to reduced bone quality in osteoporotic bones, it is often difficult to achieve sufficient anchoring for implants [1-3]. Pertrochanteric femur fractures are strongly associated with existing osteoporosis. As a result, most of implant-associated complications in pertrochanteric femur fractures are attributed to a cut-out [4]. To minimize the failure rate in osteoporotic bones, in addition to the further development of existing osteosynthesis systems, implant augmentation with cement is a promising option [5]. In this context, polymethylmethacrylate (PMMA) is used for the augmentation of osteosyntheses for osteoporotic fractures and has shown promising results in the treatment of proximal femur fractures in vitro biomechanical and in vivo clinical studies [5-7]. However, due to certain negative material properties, there are reservations regarding the use of PMMA for the treatment of fractures in limbs. These reservations are in particular the absence of biodegradability with no remodeling of the PMMA and the surrounding bone [8]. The inhibition of fracture healing due to interposed PMMA [9]. More difficult conditions when removing metal and problems removing the PMMA in the event of revision surgery [10]. Furthermore there is a risk of thermal necrosis when using PMMA [11]. The resorbable bone substitution material calcium phosphate cement (CPC) is an alternative for the augmentation of osteosyntheses [12,13]. A further development of this is paste-like, ready-to-use CPC. A great advantage of paste-like CPCs is the fact that they can be applied immediately with the associated reduction of intraoperative time and their biomechanical strength compared with conventional CPCs. The main advantage of CPC over PMMA is the biocompatibility and corresponding resorbability. The pores in the sub-micrometer and micrometer range that arise when CPC sets result in osteoconductivity and thus facilitate fracture healing and bone remodeling. The lower exothermic curing reaction compared with PMMA with the potential addition of sensitive biological molecules, growth factors, and drugs are also advantageous [8]. Previous in vivo and in vitro studies have shown promising biomechanical results of CPC-augmented osteosynthesis methods, especially in osteoporotic bones [14]. However, recently published reviews indicate a need for additional studies before the conclusive evaluation of CPC augmentation of fractures of the proximal femur [15]. The aim of this study was thus the biomechanical evaluation of a resorbable, paste-like, ready-to-use CPC for the augmentation of the Targon PFT® intramedullary nail system compared with the non-augmented version on a defined pertrochanteric fracture model.
Eight pairs of freshly frozen cadaver bones were used for the tests. No additional permission from the ethics commission was required, as the donors had given their consent to the use of their organs for scientific research while they were still alive. The samples were stored at 20° C prior to use to avoid protein and collagen degradation and were thawed to room temperature before the start of the test [16].
The bone mineral density (BMD) of each femoral head was measured according to our standard protocol [2]. The measurement was made on a 256-slice CT scanner (SOMATOM Definition Flash, Siemens Medical Solutions, Germany); the analysis software was IMPAX EE (Version R20 XV SU1, Agfa HealthCare, Germany). For calibration, a density phantom (Siemens Osteo reference phantom, Siemens Medical Solutions, Germany) was used to convert the Hounsfield units (HU) to the corresponding bone density values (BMD in mg/cm3). For every femur, 3 standardized regions of interest (ROIs) in the femoral head along the axis of the femoral head were selected manually at intervals of 2 mm; inclusion of the cortical bone and bone cysts was carefully avoided. A mean value was calculated for every femur from the Hounsfield units (HU) of the 3 ROIs.
The standardized fracture model was that of an unstable ptrochanteric fracture type 31 A2.3 of the AO classification [4]. The osteotomy was conducted after implantation of the nail. The first osteotomy line was 0.5 cm below the lesser trochanter to the greater trochanter. The second osteotomy corresponded to the line 0.5 cm proximal to the lesser trochanter to the extension of the middle of the femoral shaft.
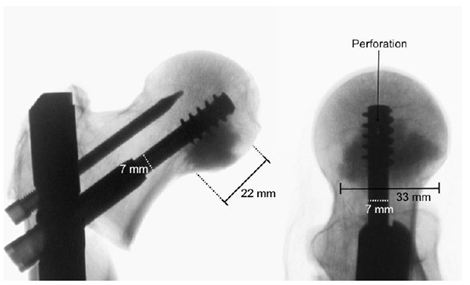
The Targon® PFT (Aesculap AG, Tuttlingen, Germany) used in this study is approved for the treatment of stable and unstable trochanteric femoral fractures. In the study, the standard nail with length of 220 mm, a diameter of 16.5 mm, and a CCD angle adjustment of 130° was used. For augmentation with CPC, standard telescopic screws of the Targon System have been perforated by the manufacturer (Aesculap AG) over the full length, with two additional fenestrations at the head of the screw ( Figure 1).
The augmentation with CPC was randomized in the 8 pairs of femurs, with one femur of each donor pair augmented. The cement was applied using a cannula (Aesculap SR146SU, Aesculap AG, Tuttlingen, Germany) with a thread for anchoring it in the telescopic screw at the end. For each augmentation, 5.7 ml of p-CPC (InnoTERE GmbH, Radebeul, Germany) were injected. Before injection, the position of the tip of the telescopic screws was checked by X-ray; the cement discharge points had to be caudal and cranial to ensure the optimal distribution of cement around the tip of the screw (Figure 2). To guarantee that the curing reaction was similar to in vivo, the bones were stored in a water bath for 5 hours and in a heat chamber at 37° C for 72 hours as in previous studies.
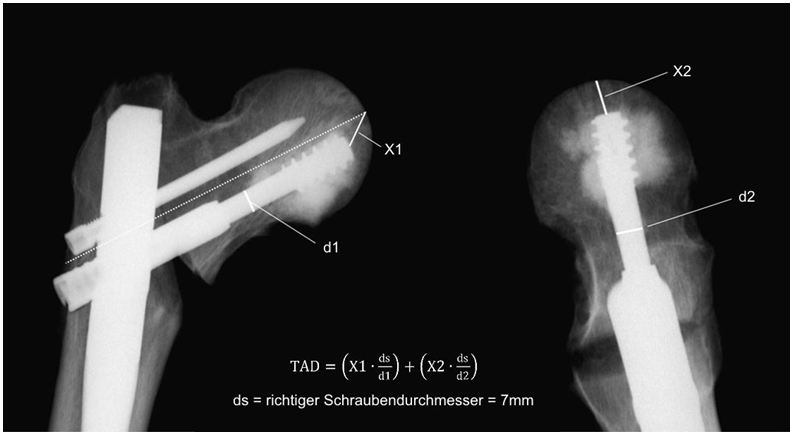
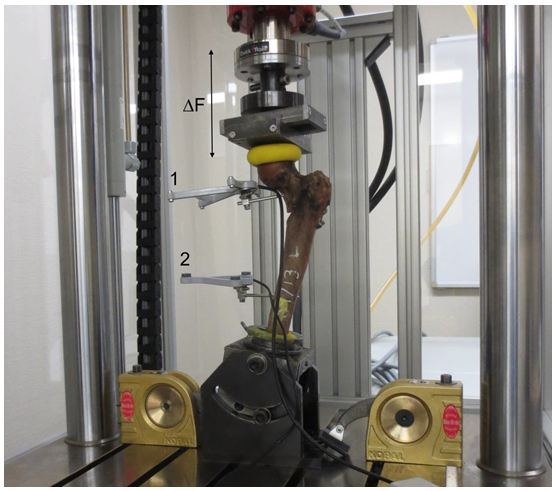
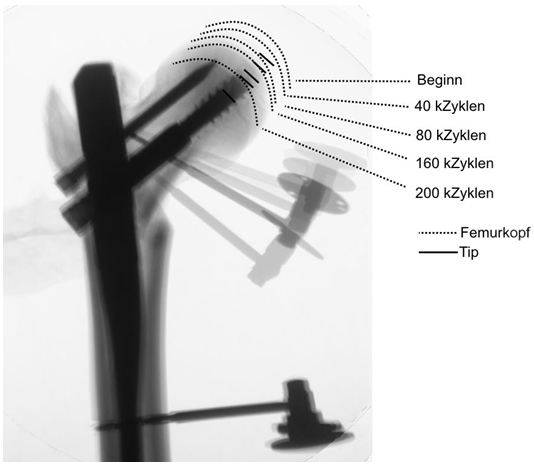
The axial transmission of force of the servo-hydraulic testing machine (Amsler HC 10, Zwick Roell, Germany) was conducted via a test dolly with two sliding plates to allow free rotation and translation (Figure 3). The femurs were subjected according to our protocol to increasing cyclic-axial loads from 0.5 to 1.75 kN for 10,000 cycles each at a frequency of 4 Hz. Then a load of 2.1 kN was applied at 4 Hz until the conclusion of the test [4]. The endpoint was defined as radiologically confirmed cut-out or reaching 200,000 cycles. The axial load exerted was analyzed using the measuring software (TestXpert® II, Zwick Roell, Germany). The angle deviation of the femoral head was measured by ultrasound (Figure 3) [4,17]. (CMS 20, Zebris® Medical, Germany). The measurements were made every 10,000 cycles at 0.5 Hz. Calibration and analysis were conducted using the measuring program Win Biomechanics (Version 0.2.6; Zebris® Medical, Germany). X-rays were used to monitor progress (Ziehm Imaging 4, Ziehm Imaging GmbH, Germany). The difference of the values at the start and after 40 k cycles was used for analyzing the tip-apex distance (TAD) (Figure 2).
The statistical analysis was made using the software SPSS Version 22 (IBM Software Group, USA). The data were tested for normal distribution using the Kolmogorov-Smirnov test. For the comparison of two groups, the t test was used for normal distribution and the Mann-Whitney U test for not normally distributed data. The contingency analysis between nominal parameters was made using the phi coefficient. All other correlation tests were conducted using a bivariate Pearson correlation. Statistical significance was assumed for p < 0.05.
The average age of the donors was 78.25 ± 11.5 years. The mean value of the BMD measured by calibrated CT was 270.6 ± 59.1 mg/cm3 within the total group; there was no significant difference between the augmented and the non-augmented control group (263.45 ± 60.43 mg/cm3 vs. 277.7 ± 60.98 mg/cm3, p = 0.482). Within the non-augmented control group, there was a significant strong correlation between the BMD and number of cycles until cut-out (r = 0.672, p = 0.034), i.e. bones with a higher BMD achieved more cycles before cut-out and bones with a lower BMD achieved significantly fewer load cycles. This BMD-dependent correlation was not observed in the augmented group (r = 0.275, p = 0.255).
In the CPC-augmented group, significantly fewer cut-outs occurred (2/8 vs. 6/8, phi = -0.50, p < 0.05) (Figure 4)
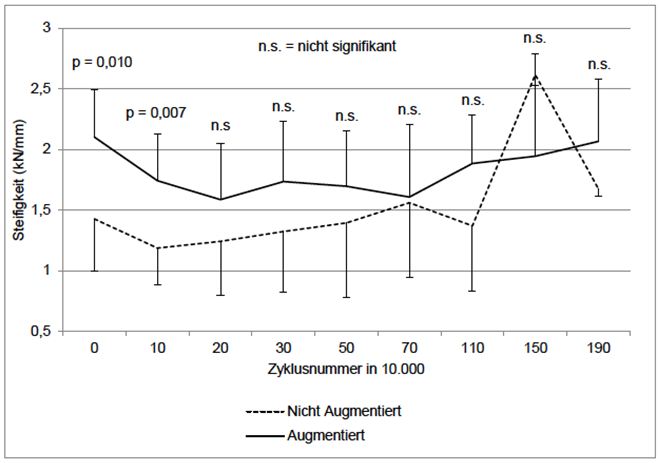
The mean stiffness at the start of the test was (2.101 ± 0.393 kN/mm vs. 1.426 ± 0.430 kN/mm, p = 0.010) and after 10 k cycles it was significantly higher in the augmented group than in the non-augmented group (2.101 ± 0.393 kN/mm vs. 1.7438 kN/mm ± 0.3873 kN/mm, p = 0.007) (Figure 5). The values approached each other over time and the differences were no longer significant. There was also a significant negative association between the stiffness at 10 k cycles and the cut-out probability (r = -0.438, p = 0.045).
Within the non-augmented control group, a significant strong correlation was found between stiffness and BMD at the start of the test (r = 0.722, p = 0.043), but this dependence of BMD and stiffness was not observed in the augmented group (r = -0.453, p = 0.260).
The augmented group had a lower axial dislocation than the control group at 40 k cycles (2.96 ± 1.47 mm vs. 3.63 ± 1.81 mm, p = 0.434) and at the endpoint (17.46 ± 6.72 mm vs. 21.21 ± 4.92 mm, p = 0.233). However, the difference was not significant. Comparable results were found for the TAD and angle dislocation. At 40 k cycles, both the TAD (0.93 mm ± 1.05 mm vs. 2.09 ± 2.24 mm, p = 0.204) and the angle dislocation (2.97° ± 1.72° vs. 3.18° ± 1.56°, p = 0.808) were lower in the augmented group than in the control group, but the differences were not significant.
The evaluation of cement distribution showed that CPC augmentation was primarily caudal to the thread of the screw. Furthermore, a lower distribution of the CPC was found around the entire thread of the telescopic screw. No cranial or concentric distribution was found. Macroscopic inspection showed homogeneous filling of the intertrabecular spaces of the cancellous bone (Figure 6).
The CPC-augmented samples were found to have biomechanical advantages over the non-augmented samples. CPC augmentation was associated with significantly fewer cut-outs. The absolute number of load cycles until the specified endpoint, axial dislocation and TAD also showed a trend toward superiority within the augmented group, however, the level of statistical significance was not reached. This is maybe an effect of sample size,
However, comparison with more than 8 pairs is very uncommon for biomechanical experiments.
Some of these observations are reflected in the existing literature. Previous prospective randomized clinical studies of unstable trochanteric fractures show non-homogeneous results for CPC-augmented dynamic hip screws with sometimes significantly fewer fracture dislocations within the augmented group and sometimes comparable results of the augmented and non-augmented osteosyntheses [18,19]. However there are always differences on the art of augmentation – with or without cement application into the fracture gap.
A key observation in our study is that CPC augmentation compensates for negative biomechanical properties of a low BMD. Within the non-augmented group, there was a significant strong correlation between BMD and cycles until the endpoint. Femurs with a high BMD thus achieved a greater number of load cycles than bones with a lower BMD. This negative effect of a low BMD was not observed in the CPC-augmented group. A negative effect of a lower BMD, for example due to osteoporosis, was thus compensated for by CPC augmentation. This observation was confirmed in the significant strong correlation between stiffness and BMD in the non-augmented control group. This negative effect on osteosynthesis stiffness, for example from osteoporotic reduction of the BMD, was not observed in the augmented group. These results are consistent with those of Yetkinler et al, who observed the cancellation of an inverse correlation between the BMD and axial dislocation in a biomechanical cadaver study of an intertrochanteric fracture model by CPC augmentation of dynamic hip screws [20]. Other biomechanical studies have shown that the effect of CPC augmentation on the stability of the implant-bone interface correlates with the BMD and thus that the lower the BMD of the treated bones, the greater the effect of augmentation with CPC is.
Comparable fracture models with intramedullary fixation devices showed a critical BMD of the femoral head, with a BMD <370-250 mg/cm3 associated with the risk of cut-out [2,21]. In the present study, the samples had BMD levels within this critical range. When the age of the donors is also taken into consideration, the cadaver bones are suitable for an osteoporotic fracture model.
This study shows a significant increase in stiffness at the start of the study and after 10 k cycles in comparison with the control group. These data were confirmed in previous biomechanical studies. However, these studies showed only an increase in stiffness at the start of the study up to maximum 1 k cycle [20,22,23]. In our study, no significant differences were found between the groups regarding stiffness after more than 20 k cycles. The most likely cause for this is the loss of the stable bond of the cement-implant interface over the course of the axial dynamic load testing with the cut-out of the screw through the CPC described above, which was confirmed by macroscopic inspection after cutting open the tested hip screws. Measured values after more than 50 k cycles were not taken into consideration in our study because increasing dislocation of the femoral head and dynamic movement of the telescopic screw over the course of the test could lead to the cortical bone of the femoral neck coming onto contact with the shaft or resting on the implant.
In conclusion it can be stated that the observed increase in osteosynthesis stiffness up to and including 10 k cycles allows earlier full loading after surgery and can thus reduce hospital stays and accelerate the rehabilitation process.
The analysis showed a more caudal distribution of CPC with slight accumulation around the thread of the telescopic screw. In an in vitro study, Sermon et al. tested different distribution patterns of PMMA in the augmentation of PFNAa blades in polyurethane foam blocks [24]. These tests showed a significant improvement in the number of cycles up to failure for all augmented bones. The best results were found for augmentation with concentric cement distribution at the tip of the screw, but no statistically significant superiority was found over caudal cementation. Transferred to our biomechanical model, modification of the telescopic screw, for example by changing the lateral fenestrations, could optimize CPC positioning and thus the biomechanical properties.
For the biomechanical test, the femurs were fixated in 13° adduction and 10° flexion. This position was selected to compensate for the functional loss of the iliotibial tract [17]. We selected an increasing dynamic loading test starting at 0.5 kN, as our previous studies have shown that immediate loading with 2.1 kN in bones with a low BMD (164-167 mg/cm) can lead to an early cut-out after 200-6000 cycles. The selected maximum load of 2.1 kN represents the force acting on the hip joint in vivo during normal walking and stair climbing found by Bergmann et al. and corresponds to approx. 300% of body weight [2].
There is an evidence of advantages of augmentation with CPC in our test model. For this, a paste-like, ready-to-use CPC was tested in a fracture model for the first time. The augmented group achieved significantly better results with respect to initial stiffness and the cut-out rate. In addition, CPC augmentation offset the negative effect of a low BMD.
Considering that cut-out is the most common complication of the treatment of a trochanteric fracture in an osteoporotic bone and that full loading can be achieved earlier because of the increased stiffness, the use of CPC appears to be an interesting treatment option. For the conclusive evaluation of CPC augmentation, in vivo testing is necessary in order to fully explore the biological advantages ‒ especially osteoconductivity and resorbability ‒ of CPC. In addition, a future in vitro study design with a direct comparison of PMMA and CPC would be useful.