In-vitro Anthelmintic Efficacy of the 80% Hydro-alcohol Extract of Myrsine africana (kechemo) Leaf on Hookworm Larvae
Background: Helminthic infections are among the most common infections in human, affecting a large proportion of population all over the world. Among the most important helminthes found in Ethiopia are Ascaris, Hookworms, Schistosomes, Strongyloides, Trichuris and Taenia saginata. Hookworms affect a large number of people in Ethiopia. Although the prevalence of hookworm and other parasitic infections are high in Ethiopia there are not enough medical services and modern medicine to treat the parasitic infection. The majority people in Ethiopia rely on traditional medicine to treat worm infestation.
Objective: The aim of this study was to evaluate in vitro larvacidial activity of the 80% Hydro-alcohol extract of Myrsine africana and determine the phyto constituents contained in the plants through the general phytochemical investigation.
Methods: A phytochemical screening for M. africanaleaf extract was done using detecting reagents for the presence of alkaloids, glycosides, steroids, saponins, flavonoids, terpenoids, and tannins among others. And in vitro larvicidal effect on the third stage larvae of hook worm was carried out using a 96-well microtiter plate assay. In-vivo acute toxicity study was also done on Swiss albino female mice.
Result: The preliminary phytochemical screening analysis revealed the presence of alkaloids, tannins, polyphenols, steroids, saponin and glycosides on the 80% Hydro alcoholic leaf extract of M. africana. The result also indicated that 80% Hydro alcoholic extracts of M. africans exhibited larvicidal activity against hookworm larva. The LC50 was 217.77 microgram per milliliter.
Conclusion: Myrsine africana appear to possess promising anthelmintic activity that may support the usage of the plant by local traditional healers to treat hook worm infection.
Keywords: Anti-helmentic; Hookworm; Myrsine africana
Abbreviations or Acronyms: EPHI: Ethiopian Public Health Institute; TMMRD: Traditional and Modern Medicine Research Directorate; SNNPR: Southern Nations Nationalities and Peoples Region of Ethiopia; OPD: Out Patient Department; DMSO: Dimethyl Sulfoxide; L3: Filariform Larva; RPM: Revolution per Minute; μg: microgram; ml: milliliter; LC: Lethal Concentration; IRB: Institutional Review Board; mg: milligram
Helminthic infections are among the most common infections in human, affecting a large proportion of population all over the world. In developing countries they pose a large threat to public health and contribute for malnutrition, anaemia, eosinophilia and pneumonia and cause malabsorbtion, diarrhoea, and other states of poor health, particularly in infants and school-age children [1-4]. Infections due to intestinal parasites are common throughout the tropics, posing serious public health problems in developing countries which may be attributed largely to socio-economic status, poor sanitation, inadequate medical care and absence of safe drinking water supplies [5]. Globally, 3.5 billion people are affected by intestinal parasites [6]. Approximately 300 million people suffer severe morbidity associated with helmenthic parasites and half of which are school-going children [7]. Helminthic diseases also pose a major health hazard to millions of livestock and cause significant economic losses in domestic and farm animals [4].
A study reported that the prevalence of intestinal parasitic infections in developing countries is almost twice those of developed countries [8]. With their high prevalence in Ethiopia, Parasitic helminthic infections cause serious public health problems [9]. More than half million visits of OPD in the country were due to intestinal parasitic infections [6]. Ascaris, Hookworms, Schistosome, Strongyloides, Trichuris and Taenia saginata are among the most important helminthes found in Ethiopia. Hookworms affect a large number of people in our country [10].
The intensive use of anthelmintics for the control of helminthic infections has resulted in the development of anthelmintic resistance, which has become a major practical problem in many countries [11].The high prevalence of intestinal parasitic infections, the lowering efficacy of current chemotherapies due to the appearance of resistant species inadequate medical services, unaffordablity and inaccessibility of modern anthelmentics particularly to the rural population have awakened the interest of medicinal plants as an alternative medicine for control of these parasites [11-15]. Thus, an urgent need for the investigation and development of effective, safer, inexpensive anti-helmenthic drugs from medicinal plants is very crucial [16-17]. Around 80% 0f the Ethiopian population relies on plant derived remedies to satisfy their health care needs [18]. Traditional herbal remedies such as the seeds of Embelia schimperi (Enkoko in Amharic ), Maesa lanceolata (Kelewa in Amharic), Hagenia abyssinica, the bulb of oxalis anthelmintica (Michamcho in Amharic), the berries of Myrsine africana (Kechemo in Amharic) and the roots of Punica granatum (roman in Amharic) etc have been used in treating tapeworm infestations for ages [6].
Myrsine africana is selected for the current study. This plant belong to the Myrsinaceae family that has been used traditionally by many of the country’s ethnic groups for treating various ailments such as tape worm [6,19]. Myrsine family, is a rather large family from the order Ericales. It is composed of more than 1000 species in over 30 genera worldwide. It is a widespread family belonging to temperate to tropical climates extending north to Europe, Siberia, Japan, Mexico, and Florida and south to New Zealand, South America, and South Africa[16]. Myrsine africana found in different parts of Ethiopia: Tigray, Gondar, Gojam, Wello, Arsi, Shewa, Welega, Kefa, Sidamo, Bale and Hararge [20]. People mix Myrsine africana dry leave powder with honey and take it orally to treat round worm and tapeworm.
Thus, the present study is intended to evaluate the in vitro larvicidal activity of the 80% Hydro alcoholic extract of Myrsine africana on hookworm larvae and identify the phytoconstitutents contained in it.
Albendazole are used as a standard drug (as positive control) during the experimental protocol and 1% DMSO was used as negative control. All the chemicals and reagents used are laboratory and analytical grade.
The fresh leaves of Myrsine africana were collected from Jimma zone located 352.4km from Addis Ababa. The plant material was identified and authenticated by a taxonomist at the Traditional and Modern Medicine Research Directorate (TMMRD) of the Ethiopian Public Health Institute (EPHI). A voucher specimen (No-2177) was deposited for future reference.
The plant leaves were washed with running tap water, dried in open air and ground to powder for extraction. Two hundred grams of Myrsine africana powdered leaves were extracted with 80% Methanol using an electrical shaker for three consecutive days. The extract was filtered using what man number 1 filter paper and concentrated by evaporation using rotary vaporizers under reduced pressure at a temperature of 40-45 °C. The filtrate obtained was then dried by steam bath at 40 °C and weighed and, finally kept in a refrigerator at 4 °C for experimental usage.
General Phytochemical screening tests were carried out for the M. africana80% crude hydro alcoholic leaf extract following procedures adopted from Harborne [21-22]. The extract was tested for the presence of alkaloids, glycosides, steroids, saponins, flavonoids, terpenoids, and tannins among others and identified by characteristic colour changes using standard procedures.
Test for Saponins: Crude extract was mixed with distilled water and heated for 5 minute, then filtered. The filtered solution was shaken vigorously for 2 minute. Foam that doesn’t disappear was taken as indication of the presence of saponin in the extract.
Test for Polyphenol: Test solution was treated with few drops of a mixture of 1 ml each of 1% FeCl3 (ferric chloride) and 1% K3Fe (CN) 6 were added to 2 ml of the aqueous solution of the extracts. Formation of green or blue color was taken as an indication of the presence of polyphenols.
Test for Glycosides: Crude extract was macerated in distilled water to form a thick mass. A piece of filtered paper was dipped in 1% Ag picric acid and 10% Na2CO3 and then suspended above the thick mass. Formation of brick red colour on the filter paper indicates the presence of cynogeneic glycosides.
Test for Alkaloides: Crude extract was mixed with 10ml of 1%HCl (hydrochloric acid) and heated for 30minute. It was cooled and filtered. To 1ml of the filtrate, 5 drops each of Mayer`s and Draggendorff’s reagents were added. Formation of white, yellow orange and white precipitate respectively indicated the presence of alkaloids in the extract
Test for Tannin: 2ml of ethanol extract was mixed with a few crystals of sodium nitrate. 3 drops of 0.1N HCl was added to extract solution. The formation brown colour indicates the presence of tannin.
Test for Steroids: 1.5% vaniline spray: The spray reagent is prepared by mixing equal volume of ethanol and sulphuric acid. Spray the TLC plate with reagent followed by heating in an oven at 90-100 ºC for 2-3 minute. Brown spot indicates the presence of steroid.
Standard solution: Albendazole (200,100, 50, 25 and 12.5 μg.mL-1) prepared from 20,000 μg.mL-1of previously prepared stock solution) was administered as standard solution.
Test solution: Different concentrations (1000, 500,250,125 and 62.5 μg.mL-1) of methanol leaves extracts of M. africanawere prepared from 100,000 μg/ml of previously prepared stock solution. All the extracts and the standard drug solution were freshly prepared before starting the experiments.
Parasite acquisition: Hookworm parasites used in this study were obtained from a source population of 200 patients that visited Cheha Health Center Laboratory, Southern Nations Nationalities and Peoples Region of Ethiopia (SNNPR) located 180 km away from Addis Ababa. The site was chosen because it is known to be endemic for hookworm disease and has favorable condition. Diagnosis of the parasite was undertaken by standard microscopic techniques. A faecal sample that tested positive for both Strongyloides stercoralis and hookworm was excluded from the study. The diagnosis of the parasite was cross checked by two Laboratory technologists for confirmation.
Larva preparation: Harada-Mori technique was used to hatch the hook worm larva as follow; 1 gm of faeces was placed on Whatman filter paper No.1 and placed in a 15-ml Centrifuge tube containing 4 ml of distilled water was added to the bottom of the tube. To avoid fungal contamination Nystatin, an anti-fungal agent 10mg/ml was prepared and three drops of this solution was added to each tube [23]. The Tubes were kept for approximately 10 days at room temperature. The larva examination was commenced on the fifth day. To obtain enough number of larvas, the fluid was concentrated by low speed centrifugation (2,000 rpm for 10 minutes). The numbers of larvae present was ascertained by counting an aliquot. The volume was adjusted to achieve a concentration of one larva per microliter.
in vitro larvicidal Assay: The assay was carried out using a 96-well microtiter plate following methodology described by Gill [24]. Stock solutions of 80% methanolic crude extract of M. africanaand albendazole were prepared at 100,000 and 20,000 microgram/ml, respectively, in 1% DMSO and were serially diluted by two-fold to produce a series of dilutions. Aliquots (2μl) were added to 2% molten agar in a total volume of 200μl in individual wells of a 96-well micro titer plate. The final drug concentrations in the assay plates consisted of two-fold serial dilutions starting at 1000, 500, 250, 125 and 62.5 μg.mL-1for the plant and 200, 100, 50, 25 and 12. 5 μg.mL-1 for the standard drug Albendazole. 1% DMSO was used as a negative control. Approximately 30 larvae (in30μl of distilled water) were added to each well, and the plates were maintained at a constant temperature of 25ºC for 2 days. Then the larvae were stimulated to move by addition of 40μl of water warmed to 50 ºC to each well; Individual larvae moving with a smooth sinusoidal motion were considered to be unaffected by the test substance and counted under a microscope. All assays were performed using triplicate assay wells at each drug concentration.
In- vivo acute toxicity study: Swiss albino female mice with a weight range between 20 and 25 g were divided into two groups of six test animals each. Four hours prior to the experiment, the animals were deprived of food for while water was given ad libitum. Mice in the negative control group received saline while the experimental group received a limit dose of 2,000 mg/kg of the 80% methanolic crude extract of M. africanaorally. All animals were then strictly observed continuously for 24 hours, with due attention paid to the first 4 hours after treatment. Any overt signs of morbidity and mortality were recorded. The animals were kept for continual observation for up to 14 days thereafter [25].
The dose-response data was analyzed using non-linear regression model (sigmoidal dose-response, GraphPad Prism; Graph Pad Software, Inc., San Diego, CA). Drug sensitivity data was expressed as lethal concentration (LC50) values (with 95% confidence intervals). This is defined as the concentration of a drug required to kill 50% of the motile worms observed in wells of the micro titer plate.
Ethical approval was obtained from the ethics committee and Institutional Review Board (IRB), Faculty of Medicine, Addis Ababa University. All the study participants of the study gave informed consent before stool specimen was collected. Those with confirmed hookworm infection were treated free of charge.
The experimental animals showed no clinical signs of toxicity and overt behavioral changes at the oral limit dose of 2,000 mg/kg the crude 80% Hydro alcoholic leaf extract of M. africana. Likewise, no mortality was recorded during the observation period of 14 days. Thus, the lethal dose (LD50) of the plant extract was beyond 2000mg/kg of body weight.
The percent yield of the extracts of Myrsine africana was determined to be 8.4% (w/w). Results of preliminary phytochemical screening analysis revealed the presence of alkaloids, tannins, polyphenols, steroids, saponin and glycosides, from the 80% methanolicextracts of M. africans. The results are presented in Table 1.
The 80% Hydro-alcohol extracts M. africans exhibited larvacidial activity against hookworm.
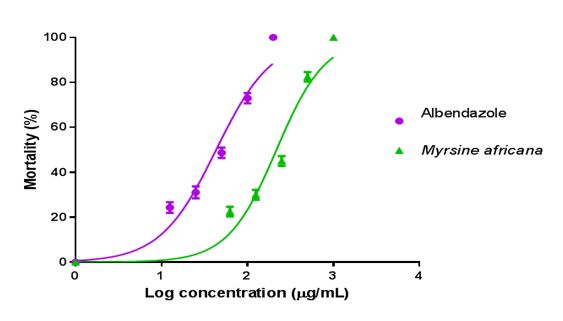
The result also indicated that 80% Hydro-alcohol extracts of M. africanaexhibited anthelmintic activity in dose-dependent manner (Figure 1).
The mean percentage mortality was plotted against the logarithm of concentrations and the concentration killing 50% of the larvae (LC50) were determined from the graph using Microsoft Excel 2013 computer software.
Traditional medicines hold a great promise as source of easily available effective anthelmintic agents to the people, particularly in developing countries. The origin of many effective drugs has been found in the traditional medicines practices and in view of this it is important to undertake studies pertaining to screening of the traditional medicinal plants for their proclaimed anthelmintic efficacy. This study tries to evaluate in vitro helminthicidal activity of M. africana[26].The use of in- vitro assay to evaluate antihelementhic activities of plants and plant extracts has two main advantages: lower cost compared to In vivo experiments and it can be conducted without interfering the physiological function of the host [27].
The acute toxicity test done on mice at limit dose of 2000 mg/kg indicated the safety of the 80% methanol extract of the plant as no overt signs of morbidity and mortality were recorded during the experimental period. In this experiment the result revealed that hydro alcoholic extract of Myrsine africana exhibited larvicidal effects on the hookworm larva. The LC50 concentration larval inhibition of the plant extract and the standard drug, albendazole were 217.77 and 43.25 μg.mL-1respectively. The concentration of extract seems a bit higher compared to standard drug, this is because extracts were crude that constituent many chemical activity. There is a need for further study to identify which specific chemical constituent has anthelmenthic activity and to understand the mechanism of action.
The larval development hasn’t been affected in the absence of the plant extracts and albendazole. Although there are no studies conducted to evaluate Myrsine africana effects on hookworm larva, several studies indicated that Myrsine africana has anthelmenthic activity [28-32]. According to a study conducted by [33]. Taenia saginata was expelled from humans after dosing with aqueous or ethanolic extracts of M. africanafruits. An in vitro study by demonstrated that alcoholic extracts of M.africana fruits were highly efficacious against the nematode Bunostomum trigonocephalum and the cestode Taenia solium [31].
Previous studies have declared the importance of some secondary metabolites such as alkaloids, glycosides, terpenoids, tannins and flavonoids for showing anthelmintic activity of medicinal plants [4,33-34]. The presence of flavonoids, glycosides, saponins, alkaloids, tannins etc in the hydro- methanolic extract of M. africanaare were responsible phytochemical constituents for demonstrating anthelmintic activity of the methanolic extract of the plants as these groups of metabolites are detected. As a result, the anthelmintic activity of this plant could be attributed to these compounds together or independently. Another previous chemical study on the plant has also resulted in the isolation of benzoquinones, anthraquinones, and a triterpenoid saponins as well as new antifungal dialkylbenzoquinone analog from the fruits of the plant [35]. The mechanism of action of active compounds of Myrsine africana is not understood. But probably these compounds have direct effect on the larvae.
The 80% Hydro alcoholic leaf extract of Myrsine africana possess anthelmintic activity that may support the usage of these plants by local traditional healers to treat hook worm infection. However, further research is required to study toxicity, mechanism of action, isolation identification and structure determination of pure chemical constituents and the effectiveness of the plant In vivo before it can be recommended for use in humans.
The authors declared no conflict of interest.