In vitro Antioxidant and Antimycobacterial Activity of Seeds of Piper longum Linn: A comparative Study
Piper longum, belonging to the family Piperaceae, have been reported to have pharmacological and clinical importance. The present study was undertaken to compare the in vitro antioxidant and antimycobacterial activity of chloroform, ethyl-acetate, hexane, ethanol, hydro-ethanol and aqueous extracts of seeds of Piper longum in order to establish the most active extract for further study. All the six extracts were screened for antioxidant activity using in vitro screening models viz. scavenging activity of DPPH radical, nitric oxide, hydroxyl radical; reductive ability and ABTS assay. Ascorbic acid served as reference standard. The extracts were also screened for antimycobacterial activities against M. smegmatis in order to authenticate the folklore claims of its use against non tuberculous bacteria. Various plant secondary metabolites were found to be present in the extracts in different proportions. Mycobacterium smegmatis (non-tuberculous bacteria) was found to be sensitive against chloroform (PC), ethanol (PE), ethyl-acetate (PEA) and hexane (PH) extracts. The mean minimum inhibitory concentration (MIC) of different extracts were in the increasing order of PC>PE=PH>PEA. MIC of PC, PH, PE, PEA was found to be 8, 16, 16 and 32 mg/mL, respectively and minimum bactericidal concentration (MBC) was calculated as 20.23, 33.43, 36.23 and 64.09 mg/mL, respectively. Chloroform extract of Piper longum (PC) showed the highest in vitro antioxidant activity as well as antimycobacterial activity.
Keywords: Antioxidant activity; Antimycobacterial activity; DPPH; Hydroxyl radical scavenging activity; Nitric oxide scavenging activity; Piper longum; Phytochemicals
Tuberculosis (TB) is a chronic infectious disease caused by a set of closely related mycobacterial strains such as Mycobacterium tuberculosis, M. bovis, M. africanum and others, known collectively as the M. tuberculosis complex (MTC) [1]. M. tuberculosis is responsible for more human mortality than any other single microbial species [2]. According to estimates by World Health Organization (WHO), almost 9 million new cases and 1.4 million TB deaths occurred in 2011 [3]. Although there are effective anti-tubercular agents, the misuse of these drugs in addition to inconsistent or partial treatment have led to the development of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB). The resistant strains coupled with drug hepatotoxicity and lengthy therapy paved the way for TB therapeutic crisis [4-7]. This situation presently acts as a serious challenge to the health care system. Thus it becomes necessary to prioritize the search for new antimycobacterial agents worldwide [8,9]. A number of extracts and isolated compounds from plants are being tested in this context. The literature reports the antimycobacterial activity of many classes of natural products such as alkanes, phenolics, acetogenic quinines, flavonoids and triterpenes [10, 11]. Several researchers have studied the possible use of certain plant extracts in the treatment of TB [12].
Phytomedicines have been utilized in the treatment of infectious diseases since the dawn of medicine [6]. The success of plant products in drug discovery can be attributed to their chemical diversity, the biological pressure to create bioactive molecules, the structural similarity of the protein targets across many species and so on [13]. Phenolics are the largest group of phytochemicals that account for the antioxidant and antimicrobial activities in plants or plant products [14]. Antioxidants decrease oxidative stress and minimize the incidence of pathological conditions caused by the oxidants. The generation of oxidative stress is harmful to the body and may cause peroxidation of membrane lipids leading to loss of membrane integrity and cell death, denaturation of proteins including enzymes, ion channels and strand breakage in DNA [15]. Thus antioxidant based drug formulations are instrumental in the prevention and treatment of complex diseases like atherosclerosis, stroke, diabetes, Alzheimer’s disease and cancer [16]. The important advantages in using medicinal plants are that they are easily available, yield profound therapeutic benefits without any adverse effect and are inexpensive treatment in comparison to their synthetic alternatives [17].
Piper longum Linn. (Piperaceae) is widely distributed in Assam, Tamil Nadu and Andhra Pradesh in India, Malaysia, Indonesia, Singapore, Sri Lanka and South Asian regions. The pungent fruits are considered as stomachic, laxative, anthelmintic, aphrodisiac, anti-dysenteric, carminative and appetizer. The fruits are also known for use as liver tonic, emmenagogue, abortifacient, diuretic and used for night blindness (Unani). The dried immature fruit and the root in the form of decoction were extensively used in acute and chronic bronchitis and reported to provide gradual relief in all such cases. A bioassay-guided isolation of an ethanol extract of the fruit of Piper longum L. yielded piperlonguminine, piperine and pipernonaline, as the main anti-hyperlipidemic constituents from Piper longum [18]. Pharmacological and clinical studies have revealed that piperine has CNS depressant, antipyretic, analgesic, anti-inflammatory antioxidant, and hepatoprotective activities [19]. Analgesic activity of Piper longum roots was reported by [20,21] reported the inhibition of TNF- α induced expression of cell adhesion molecules by inhibiting NF-κB activation and microsomal lipid peroxidation. Piperine has also been shown to enhance the bioavailability of several drugs like sulfadiazine, tetracycline, streptomycin, rifampicin, pyrazinamide, ionized, ethambutol, and phenytoin [22]. Isolates of Piper longum has been reported to exhibit antibacterial activity against both Gram +ve and Gram –ve bacteria ([23,24] studied the effects of various crude solvent extracts of roots, stems and leaves of Piper longum against a number of pathogenic bacteria (5 Gram+ve and 8 Gram-ve bacteria)and fungi. Larvicidal activity of Piper longum, black Piper nigrum and white Piper nigrum against Aedes aegypti was reported by [25,26] studied the antidermatophytic activity of chloroform, methanol, petroleum ether and aqueous extracts of leaf of Piper longum against Trichophyton mentagrophytes, T. rubrum, T. tonsurans, Microsporum fulvum and M. gypseum. The aqueous and ethanol extract of dried fruit of Piper longum yielded 100% efficiency against Giardia lamblia [27].
The present study was undertaken to determine the activity of Piper longum seeds against M. smegmatis, a non pathogenic mycobacteria but with sensitivity similar to M. tuberculosis in an effort to establish its folklore claim in the treatment for tuberculosis in NE India. Simultaneously, the in vitro antioxidant activity of several solvent extracts of the plant was also studied in order to determine the most active extract and also to find possible co-relation of its antioxidant activity with its antimycobacterial activity.
Sulphuric acid, chloroform, methanol, hydrogen peroxide (H2O2), O-phosphoric acid, sodium nitrate (NaNO2) were purchased from Merck India. Deoxyribose, thiobarbituric acid (TBA), trichloroacetic acid (TCA), ethylenediaminetetraacetic acid (EDTA), 2-diphenyl-1-picryl-hydrazyl (DPPH), sodium nitroprusside, gallic acid, nicotinamide adenine dinucleotide (NADH) were obtained from Sigma-Aldrich USA. Ferric chloride, sodium hydroxide, copper acetate, ascorbic acid, tripyridyl-s-triazine (TPTZ), nitroblue tetrazolium (NBT), phenazine methosulphate (PMS), potassium ferricyanide (K4FeCN6), potassium persulphate, sodium nitroprusside (SNP), Middle Brooke 7H9 broth, DMSO, Lowentein Jensen media were purchased from HIMEDIA. All other reagents were of analytical grade.
For ethanol, hydro-ethanol and chloroform extract, powdered Piper longum seeds from the stock were extracted by percolation for 72 h at room temperature with ethanol, hydro-ethanol and chloroform extracts respectively; mixture was stirred every 18 h using a sterile glass rod. The extracts were filtered with Whatman filter paper No.1 and the process was repeated three times for exhaustive extraction to ensure that no metabolites were left in the residues and then concentrated under low pressure to dryness at 35–45 °C using rotary evaporator (Buchi R-200). The dried ethanol, hydro-ethanol and chloroform extracts obtained from each plant were air-dried then packed for future use. Hexane and ethyl acetate extracts were prepared by successive solvent extraction of Piper longum seed powder in soxhlet apparatus at 68 –70 °C. To prepare aqueous extract, the seed powder was soaked in distilled water in a glass jar for 48 h at room temperature and the solvent was filtered. This was repeated 3–4 times until the filtrate became colorless. The filtrate was concentrated to dryness under reduced pressure in Rotavapor (Buchi R-200) and finally freeze dried. Recovery in terms of dry weight percentage of ethanol (PE), chloroform (PC), hexane (PH), ethyl acetate (PEA), aqueous (PA) and hydro-ethanol (PHE) extract of Piper longum were found to be 9.45, 5.43, 1.65, 8.05, 4.36, and 4.1 respectively.
The extracts were subjected to preliminary phytochemical testing of the seeds to detect for the presence of different chemical groups of compounds [28]. Air-dried and powdered materials were screened for the presence of saponins, tannins, alkaloids, flavonoids, triterpenoids, steroids, glycosides [29-31].
Determination of total phenolic content: The concentration of total phenolic compounds in the extract was determined by using line of regression equation obtained from the standard curve of gallic acid (Y = 0.123X + 0.021, R2 = 0.9991, Y = absorbance (nm), X = gallic acid concentration and R2 = correlation co-efficient) and expressed as µg of gallic acid equivalent (GAE) per mg extract [32].
DPPH Assay: In order to evaluate the free radical scavenging activity of the test samples, the change in optical density by DPPH radical was assessed [33]. The sample extracts were diluted with methanol to give different concentrations. Then 0.2 mL of DPPH was added to 2.8 mL of the extracts at various concentrations and incubated at 37 º
DPPH Scavenged (%) = [(Abscontrol– Abs test) / Abscontrol] × 100
Where Abscontrol is the absorbance of the control reaction and Abstest is the absorbance in the presence of the sample.
Nitric Oxide (NO) scavenging activity: Sample of various concentration were used to determine their effect on the NO radical scavenging activity using sodium nitroprusside generating NO system compared with their parent compound [34]. Then Griess reagent (1% sulfanilamide 2% O-phosphoric acid and 0.1% napthyl ethylene diamine dihydrochloride) was added to sample which stoichiometrically reacts to form a chromophore whose absorbance was measured at 546 nm. Ascorbic acid was used as standard.
Hydroxy radical scavenging activity (HRSA): The scavenging activity for hydroxyl radical was measured by studying the competition between deoxyribose and test extract for the hydroxyl radical generated by Fenton’s reaction [35]. The reaction mixture of the plant extract of different concentration reacting with 2-deoxy–D–ribose (28 mM), EDTA (1.04 mM), FeCl3 (0.2 mM) and ascorbic acid (1 mM). Subsequently, the mixture was incubated at 37 °C for 1 h. The damage imposed on deoxyribose due to the free radical was determined colorimetrically by measuring the thiobarbituric acid reactive substances (TBARS). Absorbance was measured at 512nm.
Reductive ability assay: Reducing power assay method is based on the principle that substances which have reduction potential, react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), which then reacts with ferric chloride to form ferric ferrous complex that has an absorption maximum at 700 nm [36].
Free radical-scavenging ability by ABTS radical: The free radical-scavenging activity was determined by ABTS radical cation decolorization assay [37]. ABTS was dissolved in water to 7ìM concentration. ABTS radical cation (ABTS+) was produced by reacting with ABTS stock solution with 2.45 ìM potassium persulfate (final concentration) and kept in the dark at room temperature for 12–16 h before use. The radical was stable in this form for more than two days when stored in the dark at room temperature. For the study of infusion, the samples containing the ABTS solution were diluted with redistilled water to an absorbance of 0.700 ± 0.02 at 734 nm and equilibrated at 30 oC. Reagent blank reading was taken. After addition of 3.0 mL of diluted ABTS solution, the absorbance was recorded exactly at 6 min at 734 nm after the initial mixing. Percent (%) inhibition was calculated using line of regression equation obtained from the standard curve of trolox. All determinations were performed in triplicate.
Different dilutions (32, 64, 128 mg/mL) of extracts of Piper longum (PC, PH, PE, PEA, PHE, and PA) were tested for its anti-bacterial activity against M. Smegmatis, a non-tuberculous bacterium. Evaluation was carried out by standard agar well diffusion method which was performed on a sterile Middle Brooke agar plate for the sensitivity of test sample [38].
Mycobacterial inoculants preparation: M. Smegmatis (MTCC) was cultured and grown on Lowentein Jensen media (LJ) and then subcultured in Middle Brooke 7H9 broth supplemented with albumin dextrose complex (ADC) at 37 oC for 14-21 days. Optical density was measured. The cultures were diluted at 1/1000 after the optical density was recorded to be 0.2-0.3 at 650nm.
Determination of MIC (minimum inhibitory conc.) and MBC (minimum bactericidal conc.): The susceptibility test was conducted by using the broth microdilution method (BMM) in 96 well microtitre plate. Different extracts of Piper longum (PC, PH, PE, PEA, PHE, and PA) were dissolved in 1% DMSO in sterile Middle Brooke 7H9 broth supplemented with ADC to obtain a concentration of 64 mg/mL. To the wells of the microtitre plate, 100 μl of medium on was added. The extracts were serially diluted two fold to achieve final testing concentrations viz. 64-0.25 mg/mL. This was followed by addition of 100 μl of Mycobacterium suspension. Rifampicin (5 μg/mL) served as positive control. Plates were covered and incubated at 37 oC for 5 days. On day 5, 10 μl of resazurin solution (25 mg/mL) and incubated at 37 oC for 24 h. The change in colour from blue to pink. For MBC determination, an aliquot of 5 μl from each well that show no change of colour was plated on 7H9 agar and incubated at 37 oC for 48 h. The lowest concentration that yielded no growth after subculture is MBC. Similar test was done for PE, PH, PEA, PHE and PA.
Estimation of half maximal effective concentration (IC50) value: It represents the amount of sample (ìg extract/mL) necessary to scavenge free radicals by 50%. This value is calculated from the graph plotting inhibition percentage against extract concentration.
Statistical analysis: Data were expressed as mean ± SE. One way ANOVA and Dunnett’s test were done to test the level of significance [39].
Phytochemical screening of all extracts of Piper longum (PC, PE, PH, PEA, PHE and PA) for the presence of alkaloids, terpenoids, diterpenes, flavonoids, tannin, steroid and glycerides are shown in Table 1.
The total phenolic content of Piper longum extracts was in the decreasing order of PC > PA > PE > PHE > PEA > PH. The total phenolic content of PC was the highest amongst all six extracts of Piper longum (Table 2).
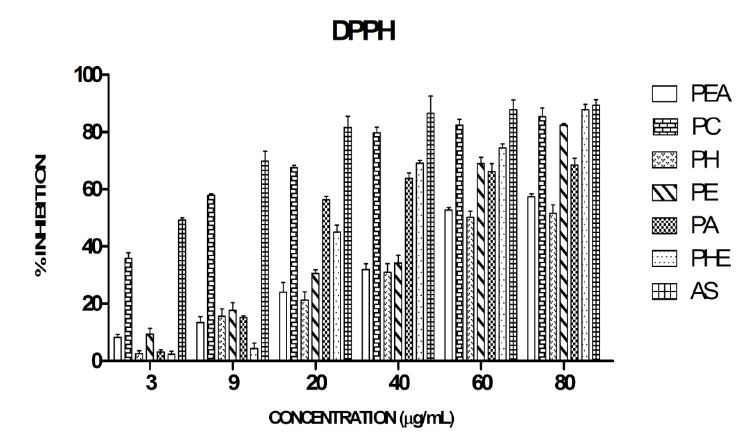
The IC50 of plant extracts increased in the following order: PC < PA < PHE < PE > PEA >PH extracts (Figure 1). The IC50 of AS, PC, PA, PHE, PE, PEA and PH were 4, 6, 19.5, 26, 50, 54 and 70 μg/mL, respectively. AS exhibited the highest DPPH radical scavenging activity. PC fraction exhibited the highest radical scavenging activity among all the six extracts.
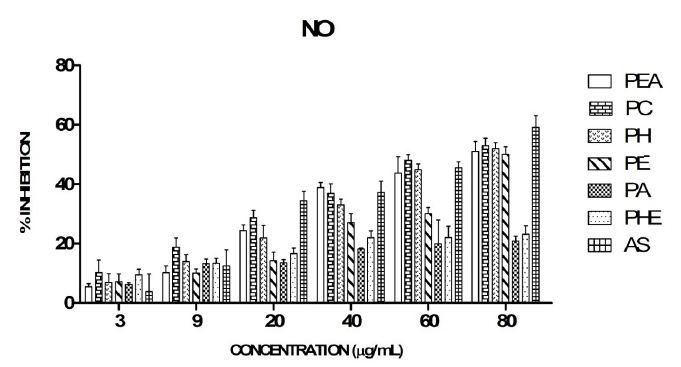
IC50 value in increasing order of different plant extracts in comparison was PC > PH =PE = PEA = PHE > PA. The IC50 of AS, PC, PH, PE, and PEA were found to be 66.5, 76, 80, 80 and 80 μg/mL, respectively. PC fraction showed the maximum inhibition of nitric oxide though lesser in comparison to AS (Figure 2).
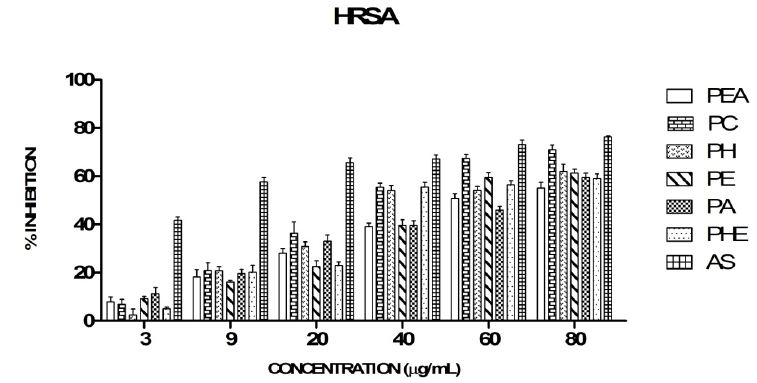
The scavenging activity of different fractions of Piper longum has been depicted in Figure 3. In this study, all the fractions exhibited appreciable scavenging activity of hydroxyl radical. The IC50 of AS, PC, PHE, PH, PE, PEA, and PA were found to be 6, 34, 39, 39, 47, 51, 60 μg/ml respectively. PC fraction has the strongest inhibition (70%) against hydroxyl radical at 80 μg/mL concentration following AS. Hydroxyl radical scavenging activities of the extracts were in the order: PC>PHE=PH>PE>PEA>PA.
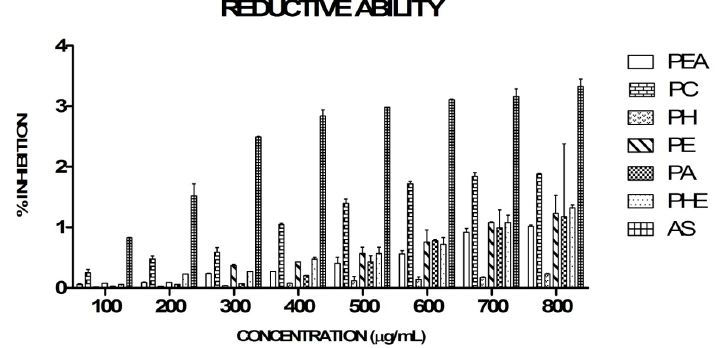
Figure 4 shows the reductive ability of different extracts of Piper longum as compared to standard ascorbic acid (AS). The reductive ability of ascorbic acid was distinctively the highest as compared to all the extracts of Piper longum. PC exhibited higher reductive ability as compared to PE, PH, PA, PHE, PEA extracts.
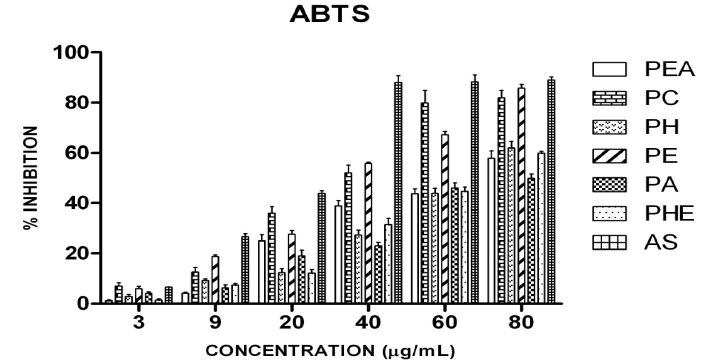
ABTS radical scavenging activity was in following order: PC>PE>PH=PHE>PEA>PA. The IC50 of AS, PC, PE, PH, PHE, PEA, PA for ABTS were found to be 20ìg/ml, 30ìg/ml, 38ìg/ml, 68ìg/ml, 68ìg/ml, 69ìg/ml, and 80ìg/ml respectively. All the extracts inhibited the free radicals in a concentration-dependent manner (Figure 5).
Different extracts of Piper longum were tested against M. smegmatis (non-tuberculous mycobacteria) using agar well diffusion method. M. smegmatis was found to be sensitive against PC, PE, PH and PEA. The zone of inhibition increased significantly with increase in the concentration of the extracts. Zone of inhibition of PC and PE varies non-significantly. Likewise, PH and PEA were also found to have non-significant inhibition against M. smegmatis. However, the activity of PC was found to be the highest when compared to all the other extracts of Piper longum (Table 3). PHE were found to be the least effective and PA showed no activity against M. smegmatis. The mean MIC values of different extracts were in the increasing order of PC>PE=PH>PEA. MIC of PC, PE, PH, and PEA was found to be 8, 16, 16 and 32 mg/mL respectively (Table 4). Here, MIC of PE and PH were found to vary non- significantly. PHE and PA both showed no inhibition of M. smegmatis. MBC for PC, PEA, PH, and PE were found to be 20.23, 33.43, 36.23 and 64.09 mg/mL respectively. MBC of PE and PHE showed non-significant difference (Table 4). Since PC showed the lowest MIC and MBC, the extract can be interpreted to exhibit the highest antimycobacterial activity against M. smegmatis.
The results of the present study demonstrated that the chloroform extract of Piper longum showed a greater content of phenolics and augmented in vitro antioxidant activity. Phytonutrients or phytochemicals are well known for their antioxidant activity. Phenolic compounds have been demonstrated to have a number of therapeutic properties such as anti inflammatory, anti-allergenic, anti-ageing, anti-carcinogenic activities which can be attributed to their antioxidant property. These properties of phenolic compounds are exerted through their ability of direct chain breaking antioxidant action by radical scavenging [40]. The screening for phytochemicals in the extracts of Piper longum seeds revealed the presence of some major phytochemicals such as flavonoids, phenolics, terpenes, alkaloids, tannins, glycosides and saponins.
Antioxidants, the first line of defense against free radicals, check the adverse effects caused due to the accumulation of free radicals in the cells by preventing or by delaying the oxidation of the oxidisable substrate [41]. The mechanism of action of these antioxidant compounds includes inhibition of the enzymes or chelating of trace elements involved in free radical production, scavenging of reactive species and up-regulation or protection of antioxidant defense [42]. Therefore, to establish the efficacy of the extracts as potent antioxidant compounds, different antioxidant models with different substrates were used for the study. The results of the present study have demonstrated that the chloroform extract have significant antioxidant activity as compared to the other extracts.
The evaluation of the antioxidant scavenging potential of bioactive compounds against DPPH is rapid, simple and easily reproducible. DPPH is a purple coloured stable free radical which decolourizes on acceptance of an electron. The antioxidant property of the corresponding extracts was estimated by measuring the bleaching of the purple colouration of DPPH at 517 nm [43]. Though PC exhibited a better DPPH radical scavenging activity, it was observed that all the extracts reduced DPPH in a concentration dependent manner. Similar concentration dependent increase in the DPPH scavenging activity of dried fruits of Piper longum was observed [44]. Similar DPPH radical scavenging activity of P.cubeba was also reported [45]. According to their findings, methanolic extract of P. cubeba exhibited an IC50 value of 11.3 ìg/mL which is almost comparable to the IC50 exhibited by PC (6 ìg/mL) in our study.
Nitric oxide takes part in the oxidation/reduction potential of a number of cells and depending on its concentration it may be involved in protection against or the induction of oxidative stress [46]. It is a key signaling molecule which acts as a potent inhibitor of physiological processes such as smooth muscle relaxation, neuronal regulation, platelet aggregation, regulation of cell mediated toxicity. Chronic expression of the radical has been implicated to play a role in carcinomas and inflammation [47]. Nitric oxide is spontaneously generated from sodium nitroprusside in aqueous solutions which reacts with oxygen (O) to produce nitrite which can be estimated by Griess reagent. Scavengers of NO compete with O leading to a decreased production of NO [48]. The extracts compete with oxygen and thus reduce nitric oxide formation. Thus, the scavenging activity of the extracts was based on the ability to prevent the NO formation. It was observed that the extracts inhibited the formation of nitric oxide in a concentration dependent manner. Concentration dependent increase in the percentage scavenging activity of nitric oxide by the seeds of P. longum was also reported [49].
Hydroxyl radical is one of the most active reactive oxygen species which reacts with the polyunsaturated fatty acid moieties of cell membrane phospholipids and cause enormous biological damage [50]. It is thus important to evaluate the scavenging potential of the extracts against hydroxyl radicals. Hydroxyl radical generated by ferric-ascorbate-EDTA-H2O2 (Fenton reaction) reacts with deoxyribose to produce thiobarbituric acid reactive substance (TBARS) which on heating with TBA forms a pink chromogen. Hydroxyl radical quenchers compete with deoxyribose for hydroxyl radicals thereby reducing the formation of the pink chromogen. Therefore, the reduction in the production of the pink chromogen on addition of the extracts was measured to estimate the hydroxyl radical scavenging potential of the extracts. It was observed that the extracts had significant scavenging effects on hydroxyl radicals that increased with the increase in concentration. Similar concentration dependent increase of hydroxyl radical scavenging activity was observed in the methanol extracts of leaves of Piper nigrum, Piper arboretum and Piper guineense [51] as observed in our study
The reducing capacity of plant extracts may serve as an indicator of the antioxidant activity of the bioactive compounds [52]. The reducing activity of the extracts could be due to the presence of reductones which have been shown to exert antioxidant effect by donating an electron. The extracts of Piper longum showed reductive ability by reducing Fe3+ ferricyanide complex to Fe2+ [53] in a dose dependent manner with the PC fraction showing better reductive ability as compared to the other extracts. Concentration dependent increase in the reductive ability was reported in ethyl acetate, water, petroleum ether and methanol extracts of Piper betel leaves [54]. These results supported our study
ABTS+. is a blue chromophore that is formed on reaction between ABTS and potassium persulfate and is reduced in the presence of hydrogen donating antioxidants [37]. The decolourisation of ABTS+. on addition of the extracts was measured to determine the scavenging potential of the extracts. This assay which is applicable for both lipophillic and hydrophilic antioxidants demonstrated that the extracts possessed potent hydrogen donating ability. It was observed that the PC fraction exhibited higher ABTS cation scavenging activity. According to reports [44] hot ethyl acetate extract of fruits of Piper longum showed high ABTS decolourisation activity.
The sensitivity of M. tuberculosis is closer to that of M. smegmatis, a non tuberculous mycobacteria and can be used for a preliminary study to select the compound with potential activity against M. tuberculosis. Screening extracts against M. tuberculosis can be hazardous due to its highly infectious nature and tedious, due to its slow growing nature [55]. Therefore, the present study conducted on M. smegmatis which has been reported to be sensitive to the action of many established anti-TB drugs and also is known to be non pathogenic to healthy humans [56], suggest that Piper longum has potential anti-TB activity. References to medicinal plants with ethnobotanical uses are mainly considered as a relevant guideline in the research of natural products. Piper longum has traditional usage in the treatment of tuberculosis. The PC fraction was found to exhibit the best antimycobacterial activity with the highest zone of inhibition and the lowest MIC, MBC. It has been well established that the antioxidant activity displayed by the phenolic compounds of plant extracts is related to its antimicrobial activity [57-59]. The PC extract exhibited the highest phenolic content. The good antioxidant potential of the PC fraction has been demonstrated by the different in vitro antioxidant screening models. Polyphenols have the capacity to link with proteins and bacterial membrane to form complexes by their hydroxyl groups or by their phenolic rings [60]. Therefore, it can be said that the superior antimycobacterial activity of the chloroform extract of Piper longum could be due to high content of interpreted bioactive substance in the extract. Furthermore, due to the fact that the cell wall of mycobacteria contain high amount of lipids, such as mycolic acid, lipophilic substances are likely to penetrate more easily into the cell [61]. Hence, extraction with intermediate-polarity solvents (e.g. dichloromethane) favors the possibility to obtain active compounds against Mycobacteria [62]. Increased membrane permeability can be considered to be a major factor in the antimicrobial action of the phenolic compounds as investigations into the mechanism of action of phenolics had revealed that it was related to the inactivation of bacterial cellular enzymes [63]. This could explain the difference in activities of the different solvent extracts of Piper longum. Chloroform as a solvent, perhaps, could extract greater amount of non polar compounds in the plant and hence could penetrate the lipid rich cell wall of mycobacteria favouring its promising antimycobacterial property. Similar results of plant extracts in chloroform solvent was observed [64] on screening nine medicinal plants used by the ethnic groups of Mexico.
This investigation supports the traditional use of Piper longum in the treatment for tuberculosis. The study demonstrated that the chloroform extract of Piper longum displayed the most effective in vitro antibacterial activity tested against M. smegmatis indicating their potential as a source of antimycobacterial drugs. Also the chloroform extract exhibited greater amount of phenolics and had significant antioxidant activity compared to the hexane, ethyl acetate, ethanol, hydroethanol and aqueous extracts. This validates the correlation of the total phenolic content of plant extracts with their antioxidant and antimicrobial properties. On the basis of these findings in vivo studies with the active extract along with the isolation and identification of bioactive compounds is presently being undertaken. (Unpublished report).
The authors are grateful to Department of Biotechnology, Government of India, New Delhi for providing financial assistance to conduct this study. The authors also express thanks to the Director of Research (Vety), CVSc, Khanapara for providing facility to conduct the research.