Intravenous Urography with Erect Film and Retrograde Pyelography in Patho-Etiology of the Loin Pain and Haematuria Syndrome Revealing the Overlooked Link with Symptomatic Nephroptosis
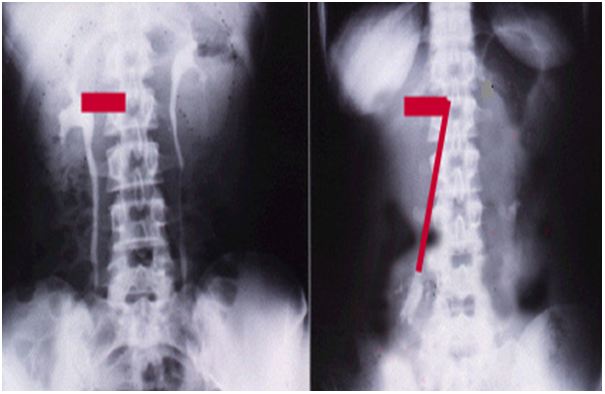
Introduction and Objectives: To report the role of intravenous urography with erect film (IVU-E) and retrograde pyelography (RGP) in resolving the puzzle of Loin Pain and Haematuria Syndrome (LPHS) by revealing its patho-etiological overlooked link with Symptomatic Nephroptosis (SN). We demonstrate that renal pedicle stretch causes renal vessels stenosis and neuro-ischaemia as evidenced by the new IVU 7 sign and the damaged renal medullary papilla shown on RGP.
Materials and Methods: Images are reported from a series of 190 SN patients. Repeated standard imaging was invariably normal, when supine. However, 190 patients demonstrated SN of > 1.5 vertebrae on repeating IVU-E. Of whom 36 (18.9%) patients developed recurrent episodes of painful hematuria for which no organic pathology was detected on all standard imaging, when supine- thus fitting the definition of LPHS. The IVU 7 sign, with its horizontal and vertical segments, represents the renal pedicle at supine and erect IVU films, respectively was used for measuring renal pedicle stretch causing renal vessels stenosis and ischaemia. The RGP demonstrated the internal renal papillary damage causing LPHS.
Results: of 190 with SN on IVU-E, 182 were females and 8 males. The mean age was 28.8, duration of symptoms 15.7 and hospital follow up 6.6 years. Patients showed no abnormality on IVU or ancillary imaging when supine. All patients showed renal drop of >1.5 vertebrae (>5 cm) on erect IVU film. Stretch/rotation of renal pedicle causing neuro-ischaemic pain of LPHS was demonstrable on the right side in 72 (37.9%) and bilaterally in 7 patients.
Obstructive complication of SN on IVU-E included ballooned renal pelvis, hydronephrosis and upper pole diverticulum. Neuro-schaemic complications induced by pedicle stretch and rotation/twist were haematuria of the LPHS affecting 36 (18.9%), auto nephropexy affecting 12 right kidneys, upper pole calyctiasis with extra-vasation affecting 28 (15.8%) right kidney and 2 bilateral that are best shown on RGP. Renal atrophy affected 4 right kidneys. Upper pole infarction affected 2 kidneys. Retrograde pyelography (RGP) demonstrated upper pole calyctiasis with extra-vasation. Surgical treatment was used in 28 patients; 10 had simple nephropexy and 18 had RSD&N for severe LPHS. Four of the patients treated with simple nephropexy had recurrence of LPHS while those who had RSD&N were all cured.
Conclusion: Upright IVU film and RGP are essential for the diagnosis of SN complicating into LPHS. The new IVU 7 sign affirms that pedicle stretch causes ischaemic nephropathy. Renal sympathetic denervation and nephropexy is curable for LPHS but simple nephropexy is not.
Keywords: Intravenous Urography (IVU); Retrograde Pyelography (RGP); Loin Pain Haematuria Syndrome (LPHS); Nephroptosis
Symptomatic Nephroptosis (SN) has been known for centuries but was disparaged >80 years ago [1,2]. The Loin Pain and Haematuria Syndrome (LPHS) reported in 1967 [3]. The use of IVU with erect film (IVU-E) and retrograde pyelography (RGP) in revealing patho-etiological overlooked link between SN and LPHS and the introduction of a new curative surgery are new discoveries [4-6].
This article is based on a prospective 10 years observational study on LPHS complicating SN [4-6]. Episodic loin pain (LP) or LPHS that has no detectable pathology is frustrating to both patient and urologist. I inherited 30 cases from my predecessor at King Khaled Hospital, Najran, Saudi Arabia. Repeated investigations and imaging including computerized tomography (CT), magnetic resonance imaging (MRI) and magnetic resonance arteriography (MRA) were all normal-being feasible only on supine posture. A patient showed me a maneuver to bring her own right kidney down to right iliac fossa and keeping it there for examination (Loin grip sign) at supine posture. Ever since I diagnosed 1.7new cases/m and found the underlying cause of SN for LPHS. The main management problem of loin pain was the lack of demonstrable pathology on repeated imaging, when supine. The underlying SN though well known was disparaged and LPHS though well documented, its existence may be doubted and both are extremely problematic to manage [3-6]
Demonstrable renal pathology of loin pain and haematuria was invariably lacking on all supine imaging of the received protocol which included CT, MRI and MRA [5-8]. Urinary tract infections (UTI) may affect a few patients during the occasional episodes but UTI, stones and organic causes play no role in the pathogenesis of LPHS. Many complex ramifying management problems of SN and LPHS are well known, but have no solutions. Some of the problems were communicated [9,10]. The illusive overlooked link of SN with LPHS was pointed out recently [4-6].
The objective of this prospective study was to answer the following questions:
– Is chronic episodic LP/LPHS genuine?
– Is there an organic pathology? How to reveal it?
– Why is the Standard Imaging Protocol (SIP) always negative?
Defining
– Nephroptosis means mobile kidney.
– Standard Imaging Protocol (SIP) as in current use.
– Upright Imaging Protocol (UIP) in which an IVU with erect film (IVU-E) is done.
Demonstrating:
– Features and complications of SN seen on UIP not on SIP
– Patho-etiology link of SN with LPHS.
Discussing:
– Reasons for disparaging SN, ignoring it by Textbooks and overlooking it in routine urological practice
– USA Authorities ignores both SN & LPHS, UK Authorities ignores SN and what the leaders choose to ignore, others do not see.
Nephroptosis is differentially diagnosed from ectopic Kidney by blood supply and mobility. In SN the kidney drops >1.5v (>5 cm) on erect IVU film causing pain with/out haematuria. In LPHS no detectable anomalies is seen on SIP. Measuring renal drop, laterality, pelvi-ureteric junction (PUJ) kink and pelvi-calyceal (PC) dilatation as evidence of obstruction on IVU E, have established renal pedicle stretch and rotation as evidence of renal neuro-ischaemia. Although SN is known, it was disparaged >80 years ago and omitted from all textbooks. SIP is constantly normal- at supine posture [5-8]. UIP (IVU-E) is rarely requested, hence chance diagnosis of SN is unlikely and diagnosis is easily missed. Many of SN features and some complications were documented >80 years ago. The link of SN with LPHS is a new discovery explaining its real patho-etiology [5,6].
Here we report the evidence that IVU-E and RGP are vital diagnostic tests for revealing the patho-etiological link of LPHS with SN.
Photographs are taken from a prospective study on LPHS and SN used to demonstrate the value of IVU-E and RGP in revealing the patho-etiology of LPHS. The IVU-E demonstrates the IVU7 sign which shows the degree of stretch in renal pedicle on erect posture which in turn causes vascular stenosis and ischaemia.
This is based on a prospective observational 10 years study of 236 patients presenting with episodic LP/LPHS. All had NO detectable anomalies or known organic causes of LP/LPHS on SIP. Of presented cases, 190 demonstrated anomalies on UIP of IVU-E and RGP. All 190 SN patients had renal drop >1.5 and up to 5 vertebrae (>5 to 17.5 cm) on IVU-E film. Most patients also demonstrated other features and complications on UIP, while SIP is negative as it is routinely done at supine (S) posture. KUB, US & IVU were normal when S. Ancillary imaging of CT, MRI, MRA and Isotope Renography (IR) were also normal at S posture. UIP is the same as SIP after adding erect (E) IVU film. IVU-S was repeated with erect film at 15 min (IVU-E). Isotope renography (IR) was done at S and sitting postures. Retrograde pyelography (RGP) with Cystoscopy was done on patients who consented.
• In order to affirm LP/LPHS is genuine we developed the following new methods
– Loin Grip Sign gripping the loin while the patient in standing position holds the kidney at ptosis position after lying supine, for examination at right iliac fossa.
– One step jump test asking the patient to jump from one step to the floor causes agony which affirms that pain is real and genuine.
• To affirm pedicle stretch causes renal ischaemia
– The IVU 7 sign, mapping the renal pedicle on IVU-S and IVU-E and measuring both demonstrate its degree of stretch and vessels stenosis.
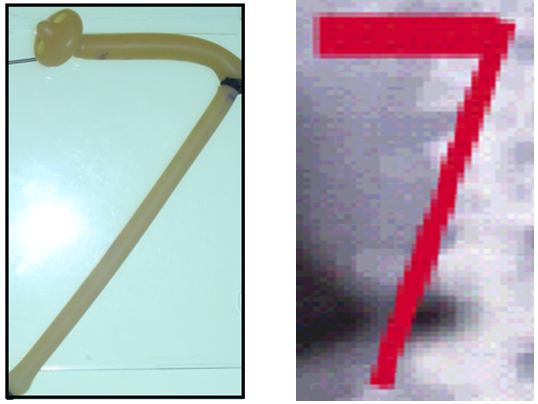
- Rubber tube stretch hypothesis shows that stretch narrows the lumen of a tube and stops flow inside its lumen.
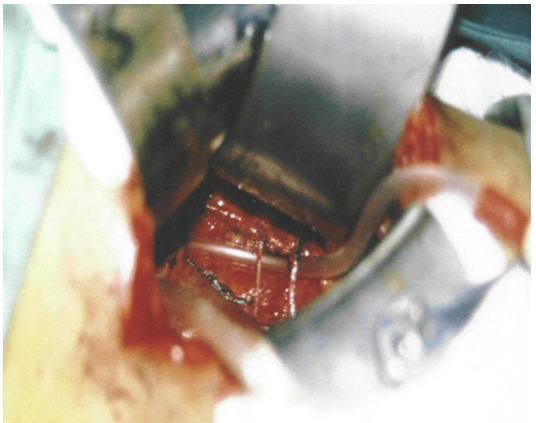
This is affirmed by the tube stretch hypothesis as well as confirmation of renal vessels stenosis on surgical exploration when the kidney is pushed to the ptosis position it becomes cyanotic. The kidney was explored by open surgery, placing the kidney at ptosed position caused stretch of renal pedicle and ischaemic cyanosis of the kidney. It was observed that renal vessel stenosis was initially intermittent but later in long standing cases it becomes organic fixed stenosis. This confirmed the IVU 7 sign and tube stretch hypothesis; representing the renal pedicle stretch ischemia and neuropathy. The kidney was explored through loin incision. The renal artery was skeletonised cutting and diathermy all surrounding nerves. Nephropexy was done by the 3 point technique
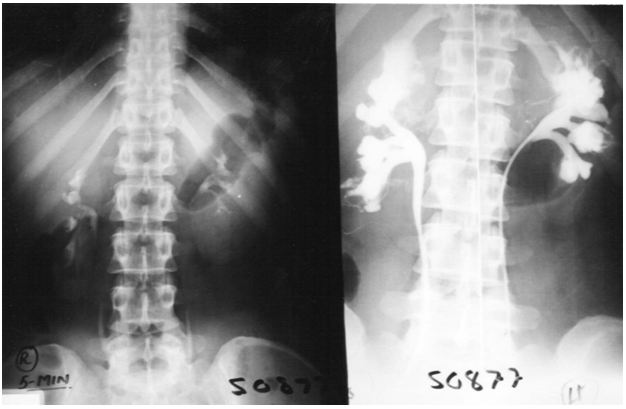
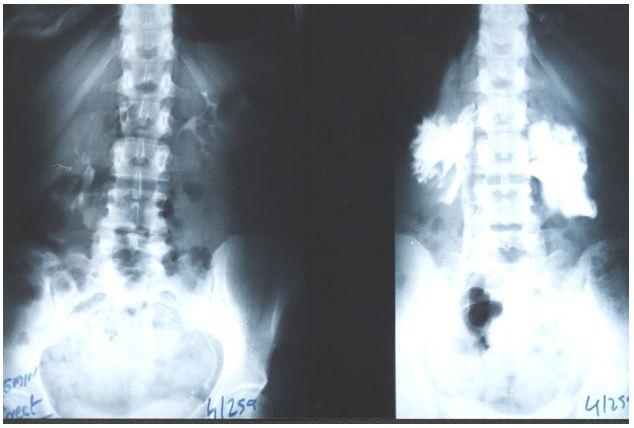
The results of this study demonstrate that IVU-E reveals the IVU7 sign that measures vessel stenosis on stretch at erect posture (Figure 1). This is further confirmed by the tube stretch hypothesis (Figure 2). Also on surgical exploration both renal artery and veins are severely stenosed in longstanding chronic cases and stenosis causes renal ischaemia (Figure 3). A normal RGP in a case of SN that does not suffer from LPHS is shown in (Figure 4).
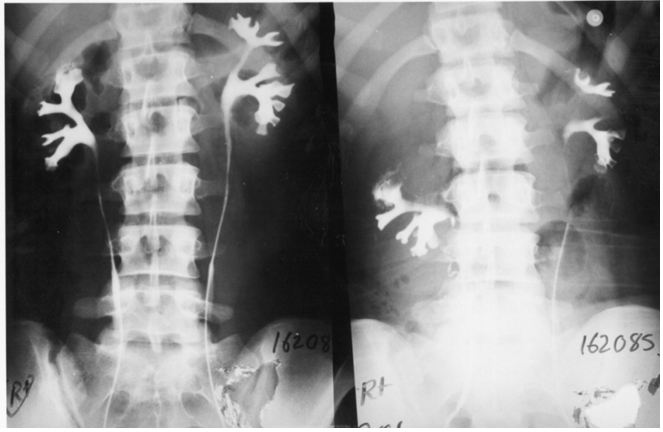
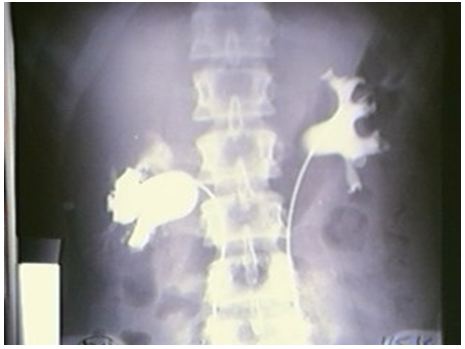
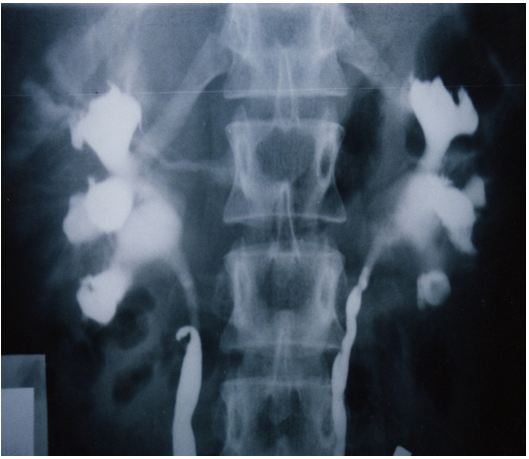
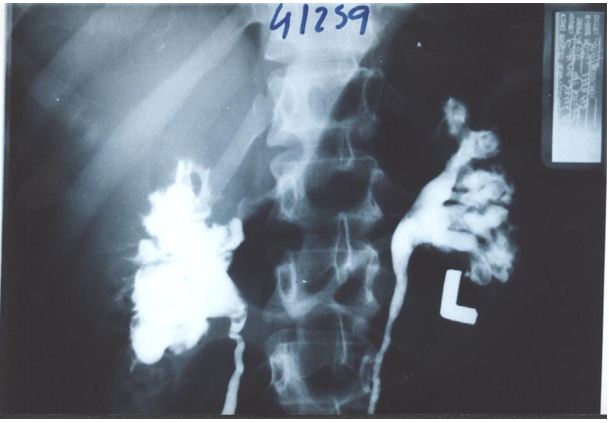
The upper pole of the right kidney is the first and most severely affected in LPHS (Figure 5 and 6). Later on the medullary damage spread to other calyxes most commonly of the right kidney. This creates calycio-venous fistula (Figure 7). That causes bleeding in one direction and contrast leakage in the other. Figure 8 shows advanced renal medullary papillary destruction in LPHS more severe on the right side (Figure 8). Compares IVU to RGP of the same patient with LPHS. The IVU looks normal while the RGP shows severe multiple bilateral papillary necrosis (Figure 9). Also CAT and MRI are normal. Again compares the IVU and RGP of the same LPHS case. The IVU looks deceitfully normal while RGP reveals the massive medullary destruction (Figure 10).
Of 190 with SN on IVU-E, 182 were females and 8 males. The mean age was 28.8, duration of symptoms 15.7 and hospital follow up 6.6 years. Patients showed no abnormality on IVU or ancillary imaging when supine. All patients showed renal drop of >1.5 vertebrae (>5 cm) on erect IVU film. Stretch/rotation of renal pedicle causing neuro-ischaemic pain of LPHS was demonstrable on the right side in 72 (37.9%) and bilaterally in 7 patients.
Complications of SN on IVU-E included both obstructive and neuro-ischaemic: obstructive complication included ballooned renal pelvis, hydronephrosis and upper pole diverticulum. Neuro-schaemic complications induced by pedicle stretch and rotation/twist were haematuria of the LPHS affecting 36 (18.9%), auto nephropexy affecting 12 right kidneys, upper pole calyctiasis with extra-vasation affecting 28 (15.8%) right kidney and 2 bilateral that are best shown on RGP. Renal atrophy affected 4 right kidneys. Upper pole infarction affected 2 kidneys. Retrograde pyelography (RGP) also demonstrated upper pole calyctiasis with extra-vasation. Surgical treatment was used in 28 patients; 10 had simple nephropexy and 18 had RSD&N for severe LPHS. Four of the patients treated with simple nephropexy had recurrence of LPHS while those who had RSD&N were all cured.
The presented photographic evidence demonstrates that IVU-E and RGP are mandatory for the proper assessment of LPHS as it reveals the underlying SN patho-etiology. The IVU-E demonstrates the IVU7 sign that measures pedicle stretch and in turn the degree of vascular stenosis that causes ischaemic damage to the kidney affecting mostly the renal medulla. The upper pole is the first and most severely affected. The right kidney is also the most commonly affected in females suffering LPHS. The RGP accurately assesses the renal damage that is proportional with the chronicity of the case.
It is worth noting that this is the first report to document the role of RGP in revealing the patho-etiology of LPHS. RGP was not previously used in the investigation of LPHS or SN. Attributing the medullary renal damage shown on RGP to mechanical issue of increasing the pressure during the injection of contrast is unacceptable. No excess pressure was used. Furthermore, RGP done on patients with LP only and without haematuria was normal while using the same technique (Figure 4).
The IVU-E was not previously used in investigating LPHS. It is observed that the whole renal pedicle is stretched which causes renal ischaemic neuropathy. Hence the operation of renal sympathetic denervation and nephropexy (RSD&N) was designed and proved 100% curable in all 18 cases who consented for this surgery. This makes all other useless surgeries in LPHS obsolete.
The IVU 7 sign and tube stretch shows that stretch to twice the normal length reduces artery or tube diameter to ½, the cross section area to ¼ and lumen flow to 1/16 (6.25%). Artery twist/ torsion induces further ischaemia. The renal pedicle contains renal artery, vein and sympathetic nerves. Thus SN initially causes functional stretch neuropathy and ischaemia explaining pain and haematuria of LPHS. It later causes organic stenosis ischameic damage to renal papillae.
This causes sympathetic neuropathy and nephropathy inducing ischaemia of SN which is initially intermittent and functional causing LP/LPHS. LPHS takes 3-4 years to complicate SN. Ischaemia organic permanent calyctaisis becomes evident on RGP: It starts at upper right calyx, common site of haematuria, spreads to other calyx of the same right kidney and affects the medullary papillae but the cortex is spared. Hence, renal biopsy may not show the damage of eroded papillae. Auto-nephropexy detected in 12 (6.3%) patients and auto-nephrectomy (Renal atrophy) in 4 patients. Focal or segmental infarctions may occur. Retrograde pyelography demonstrates the organic pyelocalyctaisis as the patho-etiology of painful hematuria, caused initially by intermittent renal ischemia of pedicle ptotic stretch or torsion [6,7] (Figure 5,6,7,8 and 9). Also PUJ kink obstruction may occur with nephroptosis which is initially functional intermittent and later cause organic PC dilatation.
The reported patho-etiology of SN pain is heterogeneous with obstruction, ischemia and neuropathy components that have reversible and organic stages. Although, these causes, and rarely UTI, may contribute to the establishment of pyelocalyctaisis, the lesion seems primarily ischemic [11-13]. The link of SN with LPHS was illusive and overlooked, particularly when the ischemic complications of auto-nephropexy, auto-nephrectomy and sympathetic nephroplegia involving the normal contra-lateral kidney occurred insidiously over many years [5,6,14]. Auto-nephropexy, made the link of SN with LPHS most illusive by erasing the evidence on renal mobility, but demonstrated that simple surgical nephropexy or spontaneous nephropexy alone may neither cure the loin pain of SN nor abort its complication into LPHS [19].
The demonstrable pathology on upright IVU, arteriography and IR are well documented on SN that was discarded long ago [1,2,13-16]. Upright imaging is currently undone and has not been reported previously in LPHS. Retrograde pyelography findings have not previously been documented in either condition. The use of IVU started early in the 20th century while clinical evidence on the genuineness of SN pain dated back to the 15th century [17]. Loin pain hematuria syndrome was reported in 1967 while Dietl’s crisis is known for centuries [2,3,17].
Organic reno-vascular complications demonstrated on conventional arteriography of SN and LPHS are of advanced cases [3,7,11,13]. The demonstrable link of SN with LPHS, other ischemic complications of infarction and atrophy “auto-nephrectomy” and the most illusive “autonephropexy” and “sympathetic nephroplegia” are reported [5,6]. Hahn [19] reported nephropexy for SN in 1881 and Black lock reported renal sympathetic denervation in 1989 [20]. The IVU 7 sign (Figure 1 and 2) and deformity of renal calyxes seen on erect IVU film demonstrate the renal pedicle stretch and torsion, respectively, which are important in explaining the neuro-ischhamic pathology of LPHS.