Lignocellulosic Biomass and Cellulolytic Enzymes of Actinobacteria
The use of white biotechnology and renewable resources as an alternative to industrial chemistry is of great interest to sustainable development and green chemistry actors. The depollution and valorization of agricultural and agro industrial waste are now major research topics; biomass plant, an abundant and renewable source of biopolymers holds a place of choice. Agricultural and forestry residues or crops are indeed one of the most important sources of first renewable on the planet. They can be used and potentially better valued. Various microorganisms, including different species of actinobacteria have biodegradation competences of organic molecules. This biodegradation function of actinobacteria is due to the variety of enzymes that they can produce.
Keywords: Lignocelluloses; Cellulolytic Enzymes; Valorization; ActinobacteriaCellulose
The carbon substrates of industrial fermentations remain one of the important things of the success plans employed. The reduction of fossil fuel resources has aroused considerable interest in renewable natural carbon resources. As such, cellulose, a major constituent of the plant cell wall has been at the center of research of a very diverse nature for the production of energy or new raw materials [1,2]. The valorization of cellulose is a considerable interest, its degradation leads to the production of chemical and energy compounds that play an important role in the carbon and energy cycle of the biosphere. Bioconversion of cellulosic waste by cellulases are established in order to value them and turn them into a mixture of more nutritious and tasty sugars, or to transform these same sugars by fermentation in alcohol used in different areas [3,4].
Actinobacteria are an important source of lignocellulose hydrolysing enzymes. They constitute considerable proportion of the soil or aquatic microflora responsible for biomass degradation in nature. The research studies on search of lignocellulose hydrolysing actinobacteria revealed the abundance and diversity of these microbes in different ecological niches. The genetic and protein studies on their hydrolytic enzymes lead to the clarification of structural and mechanism details of enzymes and their relatedness with other known lignocellulose producers and their enzyme systems. The bacteria of the Streptomyces genus are the best candidates for the production of biologically active secondary metabolites [5]. Indeed, the enzymes are after the antibiotics, the most important products of actinobacteria [6]. These species have biodegradation capacities of organic molecules as varied as recalcitrant [7]. The production of lignocellulases from all microbial sources is still quite expensive. Efforts can be made for reducing the cost of production of these enzymes using high potency actinobacteria enzyme systems with broader range of tolerance and active at diverse environmental conditions. Genetic engineering techniques can be used to construct enzyme systems with desirable characteristics. Also the studies can be stretched progressively to scale up to the industrial levels for their subsequent adoption in commercial processes.
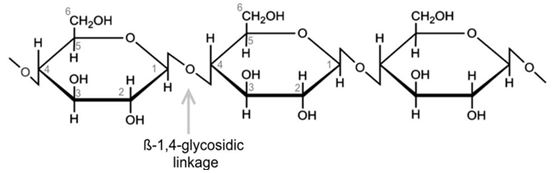
Cellulose is an important structural component of the primary cell wall of green plants. It is the most abundant natural substance produced by living organisms on earth (30% - 60% of organic carbon) (Figure 1). Cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β (1→4) linked D-glucose units [8]. The average degree of polymerization (DP) of cellulose, corresponding to the number of β-D-glucopyranose units, varies from 8,000-15,000 glucose units [9].
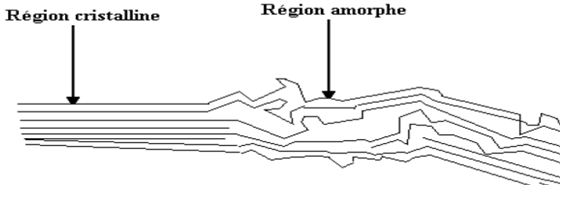
The many hydroxyl groups are responsible for the physicochemical comportment of cellulose. Depending on their position in the glucose unit, they are able to form intramolecular hydrogen bonds within the same cellulose chain or intermolecular between two different chains thus forming highly organized micro-domains. These hydrogen bonds are responsible for the formation of microfibrils in which some regions are highly ordered, crystalline and others less called amorphous (Figure 2). The highly crystalline structure is generally not accessible to the enzymatic activities, while the amorphous regions are more accessible to degradation [10]. Cellulose microfibrils are inserted in a matrix of hemicelluloses and lignin, the whole constituting the cell wall.
The second constituent of lignocellulose is hemicellulose, which is 25 to 30% of the total dry matter [18]. These polysaccharide macromolecules exhibit a great structural diversity [11]. The polysaccharide is mainly composed of: D-xylose, D-mannose, D-galactose, D-glucose, L-arabinose, 4-O-methylglucuronic, D-galacturonic and D-glucuronic acid. The various sugars are linked mainly by β-1,4-glycosidic bonds, but also with β-1,3-glycosidic bonds [10]. Hemicellulose chains have a degree of polymerization average of 150 units. Hemicelluloses are mainly amorphous and therefore have a higher chemical reactivity, explaining their higher degradation rate during wood pyrolysis. In terms of industrial applications, hemicelluloses are mainly used for the production of monosaccharides [10]. Indeed, in acidic medium, hydrolysis of hemicelluloses leads to the constituent monomers such as xylose, glucose and arabinose [12]. The alcoholic or enzymatic fermentation of the sugars thus formed, transforms them into alcohols (ethanol, butanol) and organic acids (butyric, acetic, lactic) [13,14].
Lignin is the last major part of lignocellulose, accounting for 15 to 30% of organic material. Lignin is the most important non-saccharide fraction of plant fibers. The lignin molecule is a complex of phenylpropane units, forming an amorphous structure insoluble in water. It is mainly synthesized from precursors including phenylpropanoids, the three phenols existing in lignin are: guaiacyl propanol, р-hydroxyphenyl propanol and syringyl propanol. The amount of lignin varies among different materials, in general, wood and agricultural residues contain less lignin than softwoods.
With deference to cellulose and hemicellulose, lignin is the least accessible compound for degradation. With a high quantity of lignin in the material, the resistance to degradation increases. This resistance found in lignin is a major disadvantage when using lignocellulosic material for fermentation [15]. Lignin will be a residue of all lignocellulosic ethanol production [16].
Lignin degradation is mediated by a complex of enzymes containing three principal enzymes laccases (EC 1.10.3.2), manganese peroxidases (MnP, EC 1.11.1.13), and lignin peroxidases (LiP, EC 1.11.1.14) [17,18]. Laccases are the oxidoreductases which degrade polyphenol, the principal recalcitrant component in the lignocellulose. They are extracellular inducible enzymes which employ simple oxygen as an oxidizing agent as well as cofactor.
Structural studies in several actinobacteria, have revealed presence of two cu-binding domains, rather than three [19,20].
The two-domain structure has been named as small laccase or small LMCO [19,21]. LMCOs in Streptomyces griseus, Streptomyces cyaneus, Streptomyces coelicolor, Streptomyces ipomoea, Streptomyces sviceus, Streptomyces sp., and Thermobifida fusca are active as dimers or trimmers [19,22,23].
The utilizable lignocellulosic resources are varied and come from essentially by-products of agriculture (cereal straws, corn, rape stalks, sugar cane bagasse, etc.), residues of forestry (woody substrates such as hardwood and softwoods, etc.), waste from the wood and paper industry, also from plant (triticales) or perennial species with rapid rotation (miscanthus, poplar, eucalyptus, willow).These crops represent the greatest potential for biomass and are currently a significant challenge, given their high level of production and their positive impact on the environment [17].
In this very promising future, the development of enzymatic cocktail production process is in full swing(Table 1). The main objective of the industrialists is to produce cellulolytic enzymes from the lignocellulose rather than sugar beet or sugar cane syrup or cereal starch. Such production based on lignocellulose would produce enzymes that will be competitive with those from fossil fuels and value these inexpensive raw materials, abundant, and for the moment constitute the bulk of waste industries. The chemical pathways are currently the most used to hydrolyse these lignocellulose complexes, however, the employment of specific biocatalysts becomes a realistic alternative [19,20]. However, new enzymes possessing catalytic properties and/or higher physicochemical stability are isolated from microorganisms [21,22].
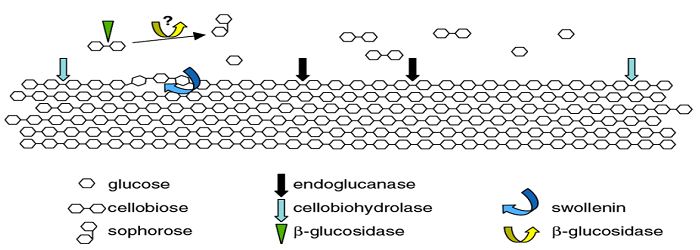
The enzymes involved in the breakdown of cellulose, are named cellulases or glycoside hydrolases. No single enzyme is able to degrade fully cellulose; the complete hydrolysis requires the synergistic action of three types of cellulases [23] (Figure 3):
Endoglucanases (EGs) (EC 3.2.1.4) that randomly cut the chains of cellulose mainly at the level of the amorphous zones generating new chain ends;
Cellobiohydrolases (CBHs) or exoglucanases (EC 3.2.1.91) which act possessively on the free ends of cellulose chains releasing cellobiose;
- β- glucosidases (BGLs) (EC 3.2.1.21), which hydrolyze cellobiose and soluble cellodextrins, into glucose.
Endoglucanases are known to randomly hydrolyze the bonds osidic, mainly in amorphous zones. They are responsible for decreasing the degree of polymerization and for creating new chain ends for cellobiohydrolase attack [24]. These enzymes possess, like cellobiohydrolases, a CBD (Cellulose Binding Domain) and a catalytic domain (CD). The catalytic domain of endoglucanases is not in the form of a tunnel, like that of cellobiohydrolases, but rather in the form of a crevice, which enables it to attack the cellulose chains everywhere and not only at the level of the ends. It is known that these attacks take place mainly in the zones with defects of crystallinity. Karlsson et al., showed that cellulose treated with phosphoric acid is made more amorphous and more hydrolyzable than avicel cellulose, which is very crystalline [25]. Endoglucanases like the majority of enzymes are inhibited by products of their actions. Macarron et al., found that the hydrolysis of chromophoric substrates was not reduced by the presence of 200 mM glucose while 50 mM cellobiose caused 10-50% decrease in the rate of hydrolysis depending on the substrate [26].
Act in a possessive way, starting from non-reducing or reducing ends of the cellulose chains, releasing cellobiose molecules. An important property of cellulolytic enzymes is their organization into domains with distinct functions. Cellobiohydrolases have two functional domains: a catalytic site (CD) that allows the hydrolysis reaction and another that allows binding to the substrate, called Cellulose Binding Domain (CBD) [27]. These two domains are linked by a peptide chain and are significantly different in size: 50 kDa for CD and 3 kDA for CBD [28,29]. The structures of the catalytic domains of cellobiohydrolases are resolved and are in the form of a tunnel [27,30]. The role of CBD is not fully understood but it would have as its first function to allow the adsorption of enzymes to the surface of the cellulose and by therefore, to increase the concentration of bound enzymes on cellulose. It would free access to glucose of cellulose chains by disrupting the hydrogen bonds between the polymer chains on the surface of cellulose structures [28]. Since cellobiohydrolases have a processive action, CBD should have a rate of progression along the cellulose fiber during hydrolysis.
However, it seems that this velocity is not a limiting element in the progression of the catalytic domain [28]. Cellobiohydrolases are known to be able to hydrolyze a solid substrate, but they also have catalytic activity on soluble oligomers [31].
Cellobiase hydrolyzes the β-glucosidic bond of cellobiose and releases two glucose molecules. Depending on its specificity, cellobiase can be active on β (1-4) oligoglucosides, but the activity decreases rapidly when the length of the chain increases.
Many authors have shown that cellobiase is strongly inhibited by its hydrolysis product glucose. This enzyme plays a key role because it reduces the inhibition of cellobiohydrolases and endoglucanases by cellobiose. It has the major role of regulating the rate of hydrolysis [23]. Microorganisms with high cellulase activity, in particular Trichoderma viridae, are deficient in cellobiases. This results in an accumulation of cellobiose and a decrease in the rate of saccharification [32]. Within each class can exist several different enzymes found as isoenzymes [33].
Synergism between enzymes is an essential phenomenon during hydrolysis enzymatic of cellulose. It occurs when the combined action of two or more enzymes leads to an activity greater than the sum of the activities of the separated enzymes. Two types of synergies have been described in the literature:
- The synergy between cellobiohydrolases and endoglucanases called Exo-Endo synergy, - The synergy between two cellobiohydrolases called Exo-Exo synergy.
Exo-Endo synergy is easily expressed because random attack by endoglucanases leads to the release of new ends of cellulose chains that become available for cellobiohydrolase attack [31].
Exo-Exo synergy is also observed but no mechanism is clearly established to explain this phenomenon and several theories are proposed. Tomme, et al., and Kim et al., explain this by the formation in solution of a CBHI/CBHII complex which would have for the former a significant catalytic activity and would allow for the latter to increase the adsorption affinity of the enzymes [34,35]. Several parameters seem to influence the synergy: - The total concentration of enzymes: more saturation of the substrate by enzymes is important, more the degree of synergy is low [36];
- The influence of the ratio of each enzyme: this is controversial, since some authors have shown that the ratio influences synergy, whereas Woodward, et al., showed that the degree of synergy was independent of this ratio [36,37];
- The morphological properties of the substrate: the increase of the crystallinity of the substrate would lead to the increase of the synergy [38].
Temperature and pH are the two main parameters influencing enzymatic activity. Enzymes denature when heated above physiological temperatures, resulting in loss of catalytic activity. Some studies have reported optimal temperatures that can reach 60 and 65 °C for the endoglucanases of Thermococcus aurantiacus, Sporotrichum thermophilia [39]. Acidic or alkaline pHs alter substrate loading by decreasing the affinity and catalytic efficiency of the enzyme, and denature the secondary or tertiary structures of cellulases and hence the active site [40]. Metal ions and chemical agents can strongly influence the cellulase activity, indeed the experimental results made by Grigorevski de Lima, et al., indicate that the CMCase activity of the strain Streptomyces drozdowiczii is strongly stimulated by Mg2+, Ba2+, Fe2+, K+ and weakly by the Na+ ion, and is inhibited by the presence of the Cu2+ ion in the mixture reaction [41]. The specificity of cellulases to different substrates also plays a role important in enzymatic activities, usually endoglucanases show a better affinity for carboxymethyl cellulose [40,42].
Actinobacteria are Gram-positive prokaryotes whose Chargaff coefficient (G+C%) is greater than 55%, generally between 60 and 75% [43,44]. Their growth is slow and can produce branched filaments 0.5-1.0 μm in diameter, smaller than fungi. The average generation time is about 2 to 3 hours [145,46]. Actinobacteria are generally chemoorganotrophs using a wide variety of energy sources including complex polymers. Several species are chemoautotrophic, using the oxidation of hydrogen as a source of energy and carbon dioxide as a carbon source [47,48]. Actinobacteria prefer a neutral or slightly alkaline pH, they are generally mesophilic, others are thermophilic, tolerate temperatures around 50 °C and can reach 60 °C. It is the most widely distributed group of microorganisms in nature, they are less predominant than other bacteria but much more than fungus [49]. However, actinobacteria are generally more frequent than almost all other bacterial groups considered individually [50]. Actinobacteria are ubiquitous microorganisms that we encounter on all natural substrates [48]. The vast majority is of telluric origin and it is from the ground that these bacteria can colonize many biotopes (air, compost, water, fodder, manure, grain, sugar cane, etc., and in various geographical areas: the extreme north, the arctic, the tropics, the highest mountain peaks and deserts, the production of odorous compounds such as geosmin and 2-ethylisoborneol is responsible for the odour characteristics of colonized soil [51-54].
Cellulose degrading actinobacteria from soil are isolated on CMC agar medium containing: (g/l): Carboxymethylcellulose (CMC), 10; KH2PO4, 4; Na2HPO4, 4; Tryptone, 2; MgSO47H2O, 0.2; CaCl22H2O, 0.001; FeSO4- 7H2O, 0.004; Agar,15 and pH adjusted to 7.0. The plates are incubated at 28 °C for 24–48 h. Zone of hydrolysis are visualised by flooding the plates with 0.1% Congo red solution and washed the plate with 1 M NaCl. Carboxymethyl cellulose hydrolysis capacity (HC value) of the isolates can be estimated by calculating the ratio of diameter of clearing zone and colony following the method of Lu, et al., [62].
The approval of the cellulase activity is done by the technique of Mandels and Weber [63]. Endoglucanase activities are estimated using 1% solution of carboxymethyl cellulose (CMC) as substrate in 0.05 M phosphate buffer (pH 7). The reaction mixture contained 0.25 ml of suitably diluted enzyme solution and 0.25 ml of substrate solution. The reaction mixtures are then incubated at 50 °C for 30 min. After 30 min the reactions are stopped by adding 1.5 ml of dinitrosalicylic acid reagent solution to the reaction mixture. The reaction mixtures are then boiled at 100 °C for 5 min. Cooling down after 5 min of boiling, O.D. are taken with spectrophotometer at 540 nm [64]. One unit of CMCase or Endoglucanase activity is expressed as 1 μmol of glucose liberated per ml of enzyme per minute. The values obtained are compared with glucose standard curve.
The regulation of cellulase production is controlled by activation and repression mechanisms, hence cellulases are known as inducible enzymes [65].
Microbial cellulase systems are either complexed or noncomplexed [30]. Complexed systems, known as cellulosomes, are characteristics of anaerobic bacteria, consisting of multienzyme complex protuberances from cell surface stabilized by dockerin and adhesion proteins. In aerobic bacteria, including most of the actinobacteria, cellulases are noncomplexed or free and are secreted extracellularly using specific secretion pathways.
Among cellulase producing actinobacteria, Cellulomonas fimi, Microbispora bispora, and Thermobifida fusca have been studied extensively [30,66].
Cellulase system of Thermobifida fusca is comprised of six extracellular cellulases (4 endocellulases and 2 exocellulases) and one intracellular β-glucosidase [66-68]. Each enzyme has a separate catalytic and carbohydrate binding domain, both linked together with a linker peptide [69,70].
A transcription factor regulating the expression of Thermobifida fusca cellulases (CelR) has been identified, and in vitro experiments indicate that cellobiose acts as an effector causing dissociation of the CelR-DNA complex [71]. The set of cellulases in C. fimi is also comprised of three endocellulases (CenA, CenB and CenD), two exocellulases (CbhA and CbhB), and a processive endocellulase CenC [70]. While these belong to the same glycosyl hydrolase families as the corresponding T. fusca enzymes, the sequences are not closely related in most cases.
Microbispora bispora also shows synthesis of six different cellulases, showing exo-exo and endo-exo synergism [66]. Genomic studies of Streptomyces sp. SirexAA-E (ActE), isolated from pine-boring woodwasp Sirex noctilio, have also shown genes for GH48 (CBH activity), GH74 (endocellulase), and CDB33 [72]. Streptomyces coelicolor consisted of 221 carbohydrate active enzymes (CAZy) or 154 glycosyl hydrolases (GHs), encoded within 8.6 billion bp long genome [72].
The extracellular cellulases are secreted by actinobacteria using either one or both of the common bacterial systems for secretion of extracellular proteins, that is, sec general secretion system and sec independent twin-arginine translocation (TAT) systems. The general secretion route catalyses transmembrane translocation of proteins in their unfolded conformation, whereas Twin-arginine (TAT) system translocates secretory proteins in their native folded state. In T. bifida both of these systems were discovered, whereas S. coelicolor mainly utilizes TAT systems for protein export [67]. Genomic analyses demonstrate that nearly all Streptomyces genomes contain carbohydrate-active enzymes (CAZy) and accessory proteins [73].
The morphological differentiation of actinobacteria is accompanied by a metabolic differentiation. In liquid medium and at the end of the biological cycle, Streptomyces produce a large number of secondary metabolites possessing a variety of chemical structures and biological activities that do not exist in any other bacterial genus. For several times, actinobacteria have been a source of various antimicrobial metabolites. Approximately two-thirds of natural antibiotics are isolated from actinobacteria, and about 75% are produced by strains belonging to the Streptomyces genus [55,56]. After antibiotics, enzymes are the most important products of actinobacteria [6]. Indeed, they are excellent producer enzymes for industrial use such as chitinases, amylases, cellulases xylanases and lipases [57-61]. Streptomyces griseus proteases are used in the food, pharmaceutical, tanning and additives in detergent industries [62]. Some species of actinobacteria degrade synthetic organic compounds which are in principle non-biodegradable, such as phenanthrene and anthracene as well as highly toxic 2,6-xylenol and widely used in industry, and which can be degraded by the Mycobacterium genus [63,64]. Actinobacteria, particularly thermophilic species and Streptomycetes, excrete enzymes that degrade cellulose [65]. Many species of actinobacteria such as Streptomyces thermoviolaceus, Streptomyces viridosporus, Streptomyces fusca have lignocellulolytic activity and are capable of producing hemeperoxidase, an enzyme that degrades lignin [65]. Enzyme inhibitors have also received attention for their potential use in pharmacology. Many types of enzyme inhibitors such as β-glucosidase and α-amylase are evidenced by Imada [66]. Some substances produced by actinobacteria are used in the degradation of hydrocarbons [67], phenol and other compounds recalcitrant [68].
Large amounts of lignocellulosic waste are generated through forestry and agricultural practices, paper-pulp industries, timber industries, and many agro industries. But, they pose an environmental pollution problem. However, the huge amounts of residual plant biomass considered as waste can potentially be converted into various different value added products including bio fuels, chemicals, cheap energy sources for fermentation, improved animal feeds and human nutrients.
Nevertheless, Actinobacteria were significant components of biomass. The prevalence of the actinobacterial genus Streptomyces could be due to the ability to synthetize enzymes, such as cellulases, which efficiently degrade lignocellulosic materials under a wide range of environmental conditions [87-89]. Actinobacteria and in particular, Streptomyces were found to be major plant biomass degrading microbes in peat swamp forests and also ubiquituously present during the composting of chestnut green waste [90,91]. The microorganism Streptomyces sp. S7 was able to grow and to produce cellulase using fruit waste as sources of carbon [92].
Prasad et al., [93] studied the effect of different cellulosic waste (vegetable peel, wood powder, and straw powder) at concentration of 1% on bioconversion process of ligninocellulosic waste by Streptomyces albospinus MTCC 8768. They obtained maximum degradation (64%) of the grated vegetable peels, followed by the straw powder (38%) and wood powder (28%).
Industrial biotechnology is one of the key technologies for the future economic development. They exploit the extraordinary properties of microorganisms and enzymes, as well as their diversity, their efficiency and their specificity, to manufacture various products.