Liver-Failure-after-Bariatric-Surgery-Clinical-Case-and-Literature-Review
The public health problem of obesity has led to a growing number of bariatric surgeries. Despite its efficacy and metabolic benefits, bariatric surgery has several complications, metabolic liver disease being one of them. Although most of the patients only experience mild to moderate disease, cases of severe steatohepatitis and progression to cirrhosis have been reported.
We present the case of a 49-year-old, morbidly obese woman who underwent a bariatric surgery (bilio-pancreatic diversion). After the procedure, she developed profuse diarrhea, persistent vomiting leading to drastic weight loss and liver failure. Liver biopsy showed steatohepatitis and fibrosis. Liver transplantation was considered but the patient improved under medical therapy in ICU. Ultimately, nosocomial infection led to septic shock and death.
Awareness of these possible bariatric surgery complications is important for its prevention, diagnosis and eventual liver transplant indication.
Keywords: Bariatric Surgery; Disease of Liver-gut Axis; Steatohepatitis
Obesity has reached worldwide epidemic proportions, and Portugal is no exception, with 59.1% of the adult Portuguese population (>20 years old) being overweight and 24.0% - obese [1]. The prevalence of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) in patients with body mass index >35 kg/m2 is estimated to be around 70% and 30%, respectively [2]. In fact, in patients undergoing bariatric surgery, NAFLD, NASH, and liver fibrosis are present in 61%, 36%, and 16%, respectively [3]. Despite improvement in histology of NAFLD after surgical intervention, some case reports have described rapidly progressive forms of liver disease [4-7].
Weight loss is the pillar of treatment of these metabolic liver diseases, that involves lifestyle changes combined with medical or surgical treatments. Bariatric surgery provides a significant and sustained weight loss to morbidly obese patients. The current European guidelines on Metabolic and Bariatric Surgery state that adult patients with BMI ≥ 40 kg/m2 or BMI 35–40 kg/m2 with co-morbidities, in which surgically induced weight loss is expected to alleviate the disorder, are candidates for bariatric surgery [2,8].
We present the case of a 49-year-old Caucasian woman with a history of morbid obesity (BMI 53.3 kg/m2) and dyslipidemia, referred to a bariatric center. She mentioned inability to lose weight with diet and exercise in the previous year. She was receiving treatment with atorvastatin and preventive low-dose aspirin. At the time of referral, she had normal liver tests (ALT 23 UI/mL, reference value (R.V.) 12-78; AST 22 UI/mL, R.V.<34; ALP 57 UI/mL, R.V. 208-378; total bilirubin 0.41 mg/dL, R.V.<1), INR (1.01), albumin (4.1 mg/dL, R.V 3,5-5,5) and platelets (275*10^9/L, R.V. 140-400). On the last abdominal ultrasound, she had a normal sized liver, with smooth capsular contour and slightly hyperechoic but homogeneous parenchyma, with no vascular or biliary changes and no visible nodularities. She did not undergo liver elastography prior to surgery. The non-invasive predictors for liver fibrosis such as APRI and FIB-4 were not compatible with advanced fibrosis (0,23 and 0,82, respectively). The NAFLD fibrosis score had an indeterminate result (-0.22 points) for advanced fibrosis.
The initial evaluation included nutritional and psychological evaluation, where it was concluded that the patient was motivated for the bariatric treatment.
The upper gastrointestinal examination before surgery showed two subepithelial lesions (3 and 5 mm) with histology suggestive of gastrointestinal stromal tumor. For this reason, the bariatric team opted for a bilio-pancreatic diversion (BPD) completed with gastric antrectomy (Scopinaro procedure). The operative protocol mentioned a normal sized liver with no gross parenchymal changes, albeit no liver biopsy was done at that time. The post-surgical recovery was uneventful. However, after the discharge, the patient did not fully adhere to the follow-up consultations, either with the surgical team or the dietitian, claiming difficulty in taking time off from work in a financially unstable situation. She also did not fully adhere to the prescribed vitamin supplementation.
Three months after surgery, the patient began to present post prandial vomiting and watery diarrhea (2-3 times a day). The barium swallow showed adequate contrast passage through a small gastric stump. She had no lesions at the upper GI endoscopy. The patient continued to be symptomatic, with progressive weakness, food refusal due to persistent vomiting, peripheral oedema and abdominal cramping. She presented to the emergency room 11 months after the surgery due to jaundice and low urinary output since the previous two weeks.
On physical examination, the patient was oriented, without flapping. She was jaundiced and dehydrated, without stigmata of liver disease. She weighed 69 kg (BMI 28.7, corresponding to a weight decrease of 46%). Her blood pressure was 78/57mmHg and the pulse was 107 bpm, with normal body temperature (36.8 ºC). The abdomen was bulky, distended, with audible bowel sounds and tender in the umbilical area. She presented with soft, bilateral oedema of the lower extremities up to the mid-thigh.
Her usual medication was only atorvastatin and she denied ingestion of any other drugs, alcohol or herbal products, unprotected sexual contacts, tattoos, recent travels, previous infection syndromes or any animal contacts.
The laboratory work-up showed normocytic anemia (hemoglobin 10.2 g/dL, R.V. 13-17.5), renal impairment with a creatinine level of 1.8 mg/dL (basal state 0.7mg/dL, R.V. 0.7-1.3). She had no leukocytosis or CRP elevation. The liver enzymes showed a mixed lesion pattern with rise in AST (502 UI/L), ALT (214 UI/L), GGT (940 UI/L, R.V.<73), LDH (1087UI/L, R.V. <190), ALP (286 UI/L) and total bilirubin of 3.1 mg/dL with elevation of unconjugated fraction (2.6 mg/dL). The INR was 2.75, with fibrinogen level of 92 mg/dL (R.V.200-400) and a factor V of 63% (R.V. 50-100). Her proteins and albumin were low (5.5 g/dL, 1.8 g/dL, respectively). Blood and urine cultures were negative.
The abdominal ultrasound showed hepatomegaly with hyper echogenic pattern suggestive of steatosis, moderate ascites and no dilation of the biliary tree or splenomegaly.
She was admitted with acute liver impairment with a MELD of 37, acute renal failure and oedema associated with malnutrition.
The liver work-up excluded autoimmune, toxic, vascular or viral causes of acute liver failure (hepatitis B, C, CMV, EBV serology were all negative). The ascitic fluid was compatible with portal hypertension and the cultures were negative. She had hypercholesterolemia (342 mg/dL, <190) and a low pre-albumin value, as well as vitamin D, folic acid and zinc deficiencies.
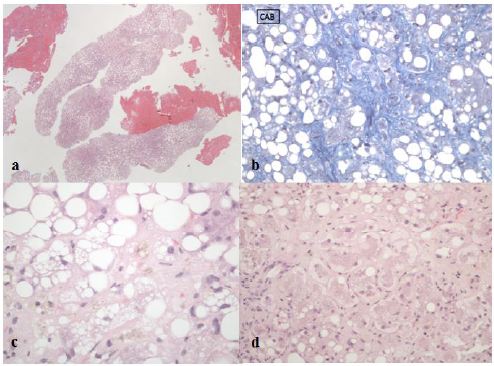
The most probable differential diagnosis was an acute liver failure in context of steatohepatitis. To confirm this hypothesis, a trans jugular liver biopsy was performed that showed hepatic venous pressure gradient of 17 mmHg and histology consistent with cirrhosis and steatohepatitis (Figure 1).
The patient was started on tailored enteric diet and micronutrient supplements.
During the first week of stay, she developed grade I hepatic encephalopathy, with a maximum bilirrubin of 12 mg/dL and lowest factor V of 32%.
Since three of the King´s College criteria were met (age, encephalopathy installed more than seven days after jaundice, non-A non-B etiology), she was referred to a liver transplantation center. However, due to improvement in clinical condition and liver function, we opted for a surveillance strategy.
Unfortunately, after a consistent improvement of her liver function, she developed a catheter-related multidrug resistant bloodstream infection, septic shock and death.
Our case represents the intricate complexity of management of obese patients.
On one hand, we fully admit the difficulty in predicting which patients will adhere to the treatment plan, either prior or after the surgery. This point favors the routine responsibility input on the patient to loose weight prior to surgical proposal. Despite being initially motivated, our patient did not fully commit to the post-surgical treatment and follow-up plan. Moreover, she did not seek medical counsel when the first symptoms of gastro-intestinal intolerance emerged, which could have prevented the fatal denouement.
On the other hand, a prior fatty liver disease, either in initial or late stages of fibrosis could have been the main driving force for the degree of liver injury observed. We fully acknowledge that it is impossible to retrospectively assess the true liver fibrosis prior to surgery, but the non-invasive scores and the macroscopic liver aspect at surgery was not suggestive of advanced liver disease.
Acute liver failure requiring transplantation is a rare complication with the new bariatric surgery techniques, estimated around 0.21% [7]. By comparison, the jejuno-ileal bypass practiced in the 1960’s had an incidence of liver failures of up to 10% [5]. Thus, for morbidly obese patients with documented liver disease, the bariatric surgeons should opt for restrictive procedures [9].
Incidence of liver failure after BPD has been reported in several case-series. The first occurrence of chronic end-stage liver disease after BPD was reported in 1992, and the first successful liver transplantation for this entity was published in 2001 [10,11].
To clarify this issue, a multicenter survey in Belgium found ten cases of liver failure referred for transplantation following bariatric surgery [4]. In nine of them, the performed surgery was BPD. In this series, two patients died on the waiting list and one was still listed. The average timespan from surgery to liver transplantation was five years. One of the seven transplanted patients required re-transplantation ten months later due to recurrence of massive steatohepatitis. This is a very interesting finding, since this was the only subject in which the small bowel transit reconstruction was postponed mere eight weeks after the liver transplant due to clinical instability.
The identified risk factors associated with liver failure after bariatric surgery are: initial BMI ≥50kg/m2, rapid weight loss in the first six months and insufficient nutritional surveillance [6]. Our patient met all these risk factors.
The etiology of the rapidly progressive liver damage following bariatric surgery is thought to be multivariable in nature. On one hand, a preexisting steatosis could progress in time and lead to liver impairment. On the other hand, a drastic weight loss and subsequent protein malnutrition triggers excessive lipolysis, which would transfer large amounts of long-chain fatty acids from visceral adipose tissue to be metabolized in the liver. Moreover, rapid bacterial overgrowth in the excluded small bowel segment could lead to mucosal injury and absorption of inflammatory cytokines and endotoxins, which in turn can lead to liver damage. Hormonal changes, infections and systemic inflammatory changes could represent other involved factors [2,6,12]. It would have been interesting to compare liver histology in our patient. Unfortunately, intra-operative liver biopsy at the time of the bariatric surgery is not part of the operation protocol in our center and there was no record of the macroscopic aspect of the liver in the surgery report. Albeit her liver tests were normal at initial evaluation, we acknowledge the fact that there was a substantial probability of prior fatty liver disease due to her high BMI.
As for the management of liver impairment and malabsorption in these cases, an adequate enteral nutrition and correction of micronutrients and vitamin deficiencies is essential. Conservative treatment with N-Acetylcysteine hasn´t shown prognostic changes and its usage should be individually tailored [13]. The correction of the nutritional deficiencies in our patient led to progressive recovery in liver function.
In case of absence of clinical and laboratory improvement with conservative measures, the malabsorptive component of the surgery should be reversed by increasing the length of the common channel loop. Baltasar suggests that the easiest way to achieve this is by creating a side-by-side anastomosis between the alimentary and the biliopancreatic loop, a so-called “kissing X” anastomosis [7]. In the cases that require liver transplantation, the reconstruction of the anatomy of the gastrointestinal tract, or at least the reversal of the malabsorptive component, should be sought. The reason for this measure lies in the potential recurrence of liver disease and altered drug pharmacokinetics of immunosuppressant agents [3].
• Although rare with the new bariatric surgery techniques, acute liver failure is a known complication of this type of surgeries.
• One of the most important risk factors for liver damage after obesity surgery is a rapid and drastic weight loss.
• Conservative measures with adequate nutrition support is imperative. In severe cases, liver transplantation should not be deferred.
• After recovery from liver damage, reversal of the malabsorptive component of the bariatric sugery should be sought.