Microleakage at Implant-Abutment Interface in Internal Conical Connection Implants, an In-Vitro Study
Microbial colonization is one of the causes of peri-implant bone loss. The type of connection and the biomechanical behavior of the Implant-Abutment complex are key in the occurrence of the aforementioned event. Internal conical connection implants have shown better fit at the Implant-Abutment interface; however, there is not any implant that has proven to block the leakage of bacteria yet. The aim of this study was to assess the outside-in micro-leakage of Escherichia coli at the Implant-Abutment (I-A) interface of two types of internal conical connection implants under unloaded conditions.
In this study, two groups of implants were tested with a total of 10 implants (5 implants per group, G1: Ankylos G2: Neodent). The Ankylos and Neodent abutments were connected according to the manufacturer’s recommendations, 15N.cm. The Implant-Abutment complexes were submerged in Brain heart infusion broth (BHI) inoculated with Escherichia coli and later, some samples were taken from the internal part of the implant at 36 hours of exposure. The bacterial penetration was assessed using Mac Farland scale, spectrophotometry, and colony-forming units CFUs.
The visual scale and spectrophotometry indicated absence of leakage in the two study groups. When using colony-forming units, there was no presence of bacteria in group 1, but group 2 had a colony-forming unit within one implant. Apart from that, no other significant difference was found between the two groups.
This study concluded that the two groups of implants are able to prevent the colonization of microorganisms under unloaded conditions.
Keywords: Microbiology; Escherichia coli; Dental Implant-Abutment design; Titanium; In-vitro
List of Abbreviations: E. coli: Escherichia coli; BHI: Brain Heart Infusion Broth; CFUs: Colony Forming Units; TIS: Taper Integrated Screw; TIF: Tapered Interference Fit; S. sanguinisl: Streptococcus sanguinis; F. nucleatum: Fusobacterium nucleatum; A. odontolyticus: Actinomyces odontolyticus; C. albicans: Candida albicans; C. glabrata: Candida glabrata
Two piece implant systems are widely used as a treatment in dental practice with high survival rates. However, bone loss during the first year after the crown placement is approximately 0.9-1.6mm due to different factors such as surgical trauma, peri-implantitis, occlusal overload, biological width formation, the patient’s immune response, and the presence of a micro-gap at the Implant-Abutment interface [1].
Dental implants have been designed with different connection types which provide particular aesthetic, mechanical and biological characteristics that, in turn, modify the geometrical configuration of Implant-Abutment interface and the loads distribution on the bone [2].
The Implant-Abutment interface is a critical area in the two-piece systems due to stress concentration as a result of the occlusal load. The formation of a microgap is caused by multiple factors: occlusal load during physiological function, manufacturing tolerance, micromotion between the Implant-Abutment connection, force-fit and form-closure between implant and abutment, off-axis occlusal contacts, protrusive contacts, Para functional forces, machining process and properties of structural material [3-6]. The size of the microgap is directly related to the penetration of microorganisms and its derivatives. They can generate complications such as modification of the contact surfaces resulting in implant wear and corrosion, inflammation of the peri-implant tissue and peri-implant bone loss [6].
The conical connection implant system was cone-in-cone designed. Aguirrebeita, et al. describe Morse taper connections as those having a small taper angle (1-3°), and locking cone system as those having a larger taper or cone angle (9-15°) [7]. On the other hand, Bozkaya, et al. classified as taper integrated screw (TIS) those that require the use of fixation screws, and tapered interference fit (TIF) those that do not use fixation screws [7,8]. These types of connections have proved to have a better clinical performance as they improve the position of the abutment, provide stability and improve the distribution of masticatory forces compared to hexagonal connections [9].
Jansen, et al., evaluated the inside-out filtration of E. coli in 9 different external and internal connections (Ankylos, Astra, Bonefit, Branemark, Calcitek, Frialit-2, Ha-Ti, IMZ, Semados) of the implant. They found that all systems, but Frialit-2 with a washer device, evidenced filtration since the first day of the trial; however, none of the implants was 100% effective blocking the bacterial leakage [10]. Similarly, Guerra, et al., assessed inside-out filtration of E. coli and Streptococcus sanguinis at the Implant-Abutment interface of external hexagonal, internal hexagonal and conical connections (Conexão Sistemas de Prótese) under unloaded conditions. They found that the bacterial counts were low and there were no significant differences in the filtration of the two microorganisms [11]. Likewise, Baggi, et al., performed the same experiment using Streptococcus sanguinis, Fusobacterium nucleatum, Actinomyces odontolyticus, Candida albicans, and Candida glabrata, and found that conical connection implants had lower filtration levels with respect to the hexagonal internal connection group [12].
To continue, Aloise, et al., assessed the filtration inside-out of Streptococcus sanguinis in two types of conical internal connections: the Bicon connection classified as a TIF (Bicon - Bicon Dental Implant, Boston, MA, USA), and the Ankylos TIS connection (Ankylos- Dentisply-Friadent, Mannheim, Germany). They found that filtration occurred in a low quantity of microorganisms in both types of implants since the second day [13]. The conical connection has shown good biomechanical performance, although, each brand individualizes the design of its conical connection by introducing particular characteristics such as changes in the conicity of the internal cone, presence of index or variation in the height of the matting zone.
The filtration has been evaluated qualitatively: dichotomous visual scale (yes / no), MacFarland scale and semi-quantitatively: spectrophotometry and colony Forming Unit (CFUs) [13-17]. For this reason, the objective of this study was to evaluate the micro-filtration of E. coli at the Implant-Abutment interface of conical internal connection implants of two different brands under unloaded conditions comparing different methodologies.
Ten implant- abutment systems were used in this in vitro study. They were divided in two groups: Group 1, Ankylos (3.5 x 11mm. Dentisply-Friadent, Mannheim, Germany) and Group 2 Neodent. Drive CM (3.5 x 11mm Neodent- Curitiba Brasil) each one with its respective standard abutment. (Ankylos regular abutment/x GH3.0) (Neodent universal abutment CM exact)
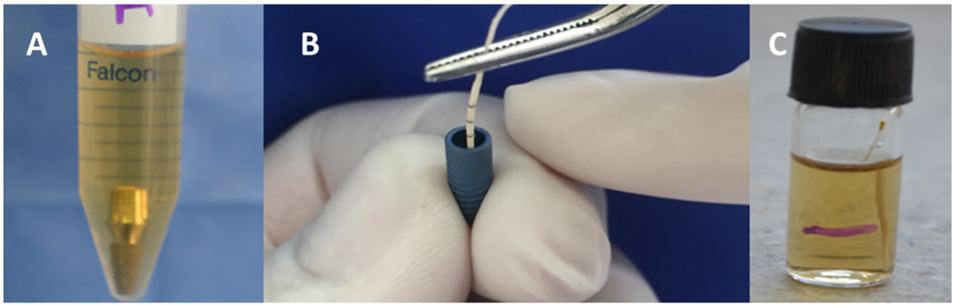
The abutments were assembled according to the manufacturer recommendations: Ankylos 15N.cm., Neodent 15N.cm. To evaluate the microbial detection techniques two Implant-Abutment replica were used [18]. As a positive control: implant system replica without assembled abutment submerged in contaminated BHI; negative control: implant system replica without assembled abutment submerged in sterile BHI (Figure 1).
The E. coli ATCC 35218 was cultured in MacConkey medium for 24 hours at 37 °C. The BHI broth was inoculated to obtain a bacterial concentration at 0.5 scale of MacFarland (1x108 colony forming units per ml) - absorbance between 0, 8-1.1 and 625 Nm [19]. The assemblies were placed into test tubes containing 5ml of contaminated BHI. They were cultured at 37 °C for 36 hours (Figure 2).
After 36 hours, the implants were removed from the test tubes and cleaned with 80% alcohol for 3 minutes and dried with sterile gauze to avoid external contamination. This procedure guaranteed external sterility without affecting the internal cell viability [20].
The abutments were removed from the implants. To minimize the possibility of contamination, the disconnection and the sampling from the inner portion of the implant with sterile paper cones were performed by two different people [21].
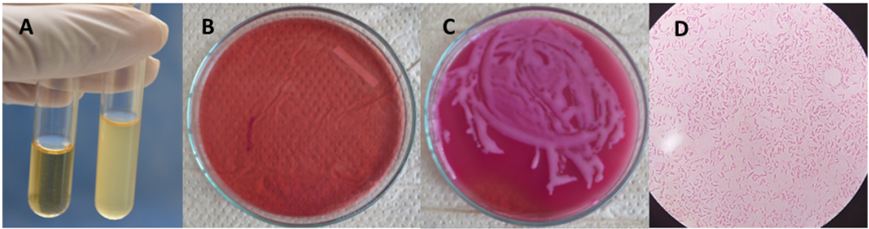
The paper cone to be used was immersed in 2 ml of sterile BHI and brought to 37 °C during 24 hours. The turbidity of the medium was observed (yes / no), a Mac Farland scale was allocated according to the degree of turbidity, and later measured by spectrophotometry. Finally, 15 μl were taken and cultured in MacConkey agar for 24 hours at 37 °C for further 24 hours to perform the UFC count. At last, the presence of E. coli was confirmed by gram staining (Figure 3).
To validate the detection techniques, the same treatment of the samples was carried out for the positive and negative controls. The data evidence that the filtration and collection conditions of the samples were carried out adequately. The positive control remained positive and the negative control remained negative. The filtration was evaluated qualitatively and semi-quantitatively. This procedure proved that in a dichotomous visual scale (yes / no), MacFarland scale, and spectrophotometry there was no evidence of bacteria in any implant. When carrying out the evaluation by means of CFUs, a Neodent implant presented a colony forming unit. When performing gram stains, it was possible to determine that the microorganisms were gram-negative bacilli. The results are shown in Table 1.
When the abutment is connected to the implant, a gap is created in the joining area between the two parts. This has been described as the micro-gap, its size varies between 1 to 150 μm, depending on the type of connection and type of abutment (prefabricated or customized) [10]. Given that oral bacteria have a diameter of 1.1 to 1.5 μm and a length of 2-6 μm on average, the micro-gap space is enough to allow the mobilization of microorganism’s bidirectionally. In this study we used E. coli, an enteric bacterium whose dimensions are similar to oral bacteria. It can grow in aerobic conditions, which makes its handling easy and predictable. Besides, this bacterium has been found in peri-implantitis lesions [22-24]. The colonization of bacteria is relevant due to its effect on the activation of inflammatory cells that lead to peri-implant tissue inflammation and subsequently bone loss.
In vitro studies of bacterial colonization are useful to approach a prognosis of the clinical behavior of this type of devices. They generate some insights that, even if they are not fully extrapolated to reality, provide evidence to make a sensible choice of the systems and components to be implemented. On the other hand, these studies may contribute to the introduction of design and manufacturing improvements from the engineering point of view.
In this study, we assessed the sealing capacity of two implant systems whose characteristics are similar in composition (Ti4Al6V), conical connection TIS, indexed components, conicity (close to 6º), and standard prefabricated abutments.
The implant filtration was assessed in a period of 36 hours from outside inwards by means of different methodologies. First, turbidity and a McFarland scales were appointed; after that, the absorbance was measured by spectrophotometry, and finally formation units of colonies were formed. The results of this study revealed that when using qualitative methodologies, the presence of bacteria was not observed. On the other hand, when colony-forming units were used, it was possible to observe a colony forming unit in a group 2 implant, unlike group 1, where no presence of bacteria was evidenced. It is possible to infer that the qualitative evaluation methods are not sensitive enough to perform this measurement, whereas the CFUs proved to be a better alternative for the measurement of bacterial invasion. Additionally, real time-polimerasa chain reaction (PCR) is a method used in molecular biology that could be more sensitive and give quantitative results [23].
In this study, the filtration found was very low, 1 of 5 implants on Neodent group showed bacterial invasion and 0 of 5 implants in the Ankylos group. The methodology used and the results are comparable with different articles that present similar results such as Aloise, et al., who assessed the inside-out filtration of two types of conical connections (Bicon TTS abutment and Ankylos abutments) using a microorganism S. sanguinisl during 14 days and evaluating the turbidity of the medium. They discovered that both implant systems presented filtration since the second day of the experiment. However, this filtration occurred only in two implants out of 10 assessed implants group [13]. Deceles, et al., carried out the same experiment for 14 days, using E. coli and Koop cone implants (TIF abutments) on the one hand, and Conexão implants, (abutments TIS) on the other. They discovered that there was bacterial leakage since day one. The Koop implants evidenced leakage in 3 out of 15 implants, and the 9 Conexão implants leaked out of 15 [25,26]. The two articles conclude that the two types of abutments exhibited filtration and no conclusive differences between the two groups [25].
Ranieri, et al., evaluated the outside infiltration of S. sanguinisl in Ankylos and Conexão conical implants, (Sao Paulo, Brazil and Osteofit, DSP Biomedical, Campo Largo, Parana, Brazil) using Scanning Electron Microscopy (SEM). They found bacterial colonization in the abutment surface and the fixation screw of three of these systems. Opposite of the Ankylos implant where the filtration was limited to the abutment body without compromising the fixation screw [26].
A different methodology was reported by Garrana, et al., They tested endotoxin filtration in external and internal conical connection implants. They concluded that the external connection implants had higher filtration values and that the Straumman and Ankylos implant brands were able to block the passage of endotoxin through the Implant-Abutment interface [27].
Hence, there are disparate results for the different types of microorganisms, the different test substances, and the different time intervals being used; the highest assessment interval went up to 14 days. Filtration occurred since the second day. As a result, it is possible to infer that the conical internal connection presents good sealing capacity. Despite this evidence, there is not any implant system able to fully prevent bacterial leakage under unloaded conditions. Nevertheless, the behavior of the leakage can vary when the implant is under loaded condition. One of the disadvantages of this study is that it was not carried out under loaded conditions.
Authors such as Koutouzis, et al., have evaluated the filtration of E. coli in conical connection implants (Implant One Fixtures, 4.0 x 12 mm, Custom Dental Implants, Norwalk, WI) under dynamic loads (500,000 cycles at 50N). They conclude that dynamic loads affect the potential of microorganism’s invasion and that filtration is very low in this type of implants [22]. Therefore, it is recommended to conduct studies where the implant behavior is assessed under dynamic load conditions, and different exposure times are tested as well.
Having in mind the limitations of this study, it is possible to conclude that the two groups of implants are able to block the leakage of E. coli through the IA interface under non-loaded conditions. It is advisable to perform the tests in dynamic load condition under longer periods of time. The authors of this study claim that there is no conflict of interest among the participants of the study. Special thanks to the oral microbiology laboratory of the Medicine School at National University of Colombia.