New Reaction of Diazomethane
A new reaction of diazomethane with cyclic amide acids was discovered, and a reaction mechanism was proposed.
Keywords:diazomethane, amide acids, methyldiazonium
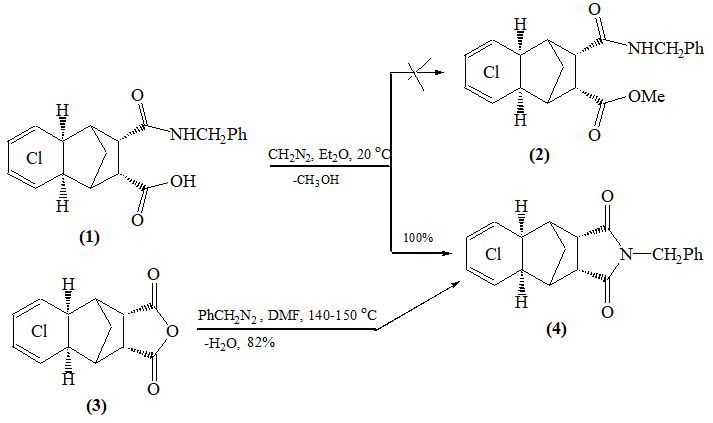
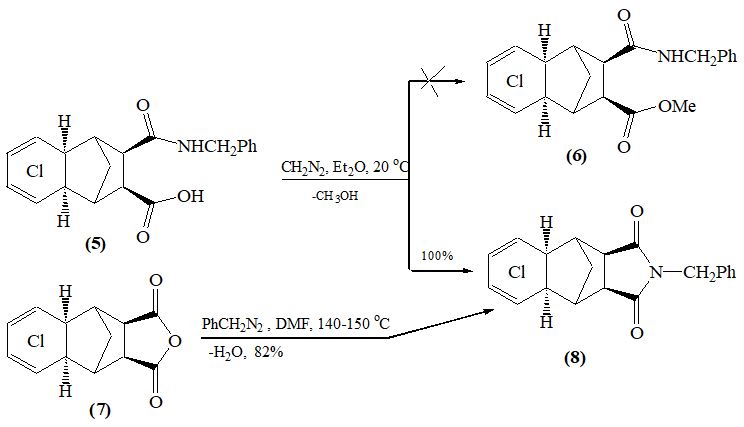
Recently, [1, 2] we found that the interaction of diazomethane with cyclic cis-amide acids (1, 5) removing water from the latter with quantitative yields, leads to cyclic endo- and exo-imides (4, 8). It should be noted that the water-taking ability of diazomethane is still unknown.
The structures of compounds (4) and (8) were confirmed by NMR ¹Н, 13С spectroscopy and counter synthesis (scheme). Note that the known methods for producing imides from amide acids require more stringent conditions, in particular, their prolonged heating in high-boiling solvents (usually in DMF, Ac2O) in the presence of water-removing agents [4].
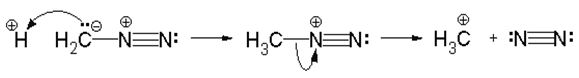
Instead of methylation of the carboxyl group, the process of water separation and the formation of an imide ring can be explained by the steric action of the reaction centers. No trace of compounds (2) and (6) was found in this reaction. It is known [3] that during protonation from diazomethane a methyldiazonium cation is formed:
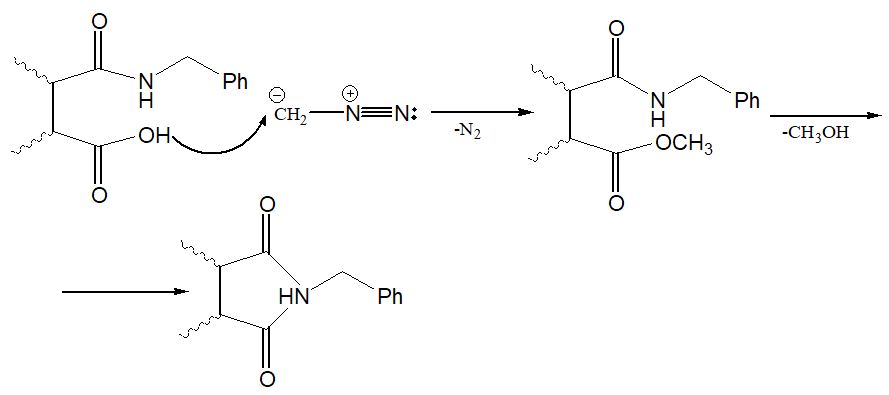
Analysis of the reaction mass showed the existence of CH3OH in its composition. Therefore, the separation of methanol and ste- reoisomeric imides (4. 8) as a result of the interaction of diazomethane with amide acids (1, 5) allowed us to suggest a mechanism according to the following scheme:
The IR spectra were taken with a Specord M-80 spectrophotometer in a liquid film and in a suspension in Vaseline oil, the absorp- tion frequencies are given in cm-1. The 1H and 13C NMR spectra were recorded on a Bruker AM 300 spectrophotometer operating at 300 and 75 MHz, respectively, the internal standard was TMS, and the solvent was DMF-d7. The chemical shifts of 1H and 13C NMR signals are shown in the scale δ, in parts per million (ppm). rel- (1S, 2R, 3S, 4R) -2,3- (N-benzylimide) -5,6,7,8-tetra- chloro-1,2,3,4-tetrahydro-1,4-methanonaphthalene-2, 3-dicarboxylic acid (4)
To a suspension of 0.02 g-mol (~ 0.9 g) of amide acid (1) in ether, an ether solution of diazomethane was added until a yellow color of the solution formed. After evaporation of the ether (at 20 °C), the precipitated white crystals were filtered off, washed with hexane, and characterized. Yield – 100%. Mp. 207-209ºС. IR spectrum (cm-1): 1720, 1780 (С = О), 1616 (С = С). ¹³С NMR spectrum (DMF-d7, δ, ppm): 177.38 s (С = О), 137.40 s (С¹ benzyl), 131.68 s (С6 and С7), 129.23 d (benzyl CH), 128.83 d (СН benzyl), 124.00 s (C5 and C8), 48.76 d (2CH), 48.21 d (2CH), 47.99 d (2CH), 42.62 t (CH2 benzyl), 38.49 t (C9). 1Н NMR spectrum (DMF-d7, δ, ppm): 1.85 d (1Н, С9Н, J 11 Hz), 2.00 d (1Н, С9Н, J 11 Hz), 2.75 m (1Н), 2.95 m (1Н), 3.10 m (2Н), 3.51 m (2Н), 4.65 s (2Н, СН2 benzyl), 7.32 m (5Н, С6Н5). Found%: C 54.32; H 3.15; Cl 32.15; N 3.05. C20H15Cl4NO2. Calculated%: C 54.42; H 3.05; Cl 32.20; N 3.18.
Similarly, was obtained rel- (1R, 2S, 3R, 4S) -2,3- (N-benzylimide) -5,6,7,8-tetrachloro-1,2,3,4-tetrahydro-1,4-methanonaphtha- lene 2,3-dicarboxylic acid (8). Yield – 100%. Mp. 215-216 ºС. IR spectrum (cm-1): 1725, 1780 (С = О), 1615 (С = С). ¹³С NMR spectrum (DMF-d7, δ, ppm): 177.35 s (С = О), 137.52 s (С1 benzyl), 131.60 s (C6 and С7), 129.04 d (benzyl CH), 128.75 d (СН benzyl), 124.20 s (C5 and C8), 48.65 d (2CH), 48.25 d (2CH), 47.84 d (2CH), 42.70 t (CH2 benzyl), 38.53 t (C9). 1Н NMR spectrum (DMF-d7, δ, ppm): 1.81 d (1Н, С9Н, J 11 Hz), 2.10 d (1Н, С9Н, J 11 Hz), 2.70 m (1Н), 2.83 m (1Н), 3.00 m (2Н), 3.58 m (2Н), 4.60 s (2Н, СН2 benzyl), 7.29 m (5Н, С6Н5). Found%: C 54.54; H 2.97; Cl 32.33; N 3.20. C20 H15Cl4NO2. Calculated%: C 54.42; H3.05; Cl 32.20; N 3.18.