Pelvic Mass with Low Fever and Weakness as The First Manifestation of a Rare Case of Retroperitoneal Follicular Dendritic Cell Sarcoma in A Young Woman: A Case-Report
Background: Follicular dendritic cell sarcoma (FDCS) is a rare neoplasm that originates from follicular dendritic cells in lymphoid tissue while retroperitoneal follicular dendritic cell sarcoma is even rarer. We present the clinical data, pathological materials and computed tomography (CT) features of a rare case of this disease.
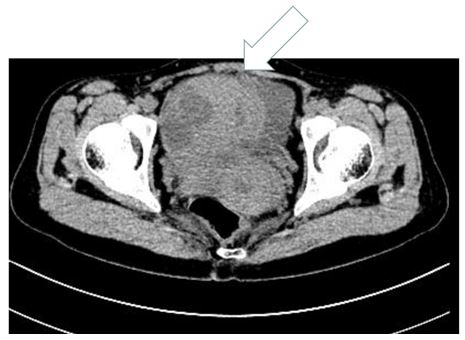
Case presentation: A 28-year-old woman presented with low fever , weakness and pelvic mass. Her laboratory results showed a high level of White blood cells (WBC) and CRP with normal elevation of CA125 and CA199. CT scan revealed a cystic-solid mass (13*10*14cm) with lineal hyper-density shadow, which was indistinguishable with the left annex. Surgical resection was performed, and the pathological diagnosis was FDCS. The patient recovered well from the operation, and went to the tumor center receiving adjuvant therapy after surgery.
Conclusions: Retroperitoneal FDCS manifested as pelvic mass with low fever, weakness and high WBC and CRP is very rare. Its CT features are not specific, and the disease should be differentiated from lymph tumors.
Keywords: Pelvic Mass; Follicular Dendritic Cell Sarcoma; Retroperitoneal; CT; Pathology
Follicular dendritic cells (FDC), also recognized as dendritic reticulum cells, are an indispensable part of B-cell follicles. Follicular dendritic cell sarcoma (FDCS), a rare neoplasm with follicular dendritic cell (FDC) differentiation, was first reported in 1986 by Monda, et al [1].Since then, more than 300 patients of FDCS have been reported worldwide [2]. FDCS is a neoplastic proliferation of spindled to ovoid cells with morphologic and immunophenotypic features similar to those of ordinary FDCs and is classified under histiocytic and dendritic cell neoplasms by the World Health Organization Classification of Tumours [3]. Approximately two-thirds of FDCS involves the lymph nodes, most commonly involving cervical and mediastinal lymph nodes. Meanwhile, one-third of cases affects extranodal organs such as the liver, the gastrointestinal tract, soft tissue, tonsils or skin [4, 5]. Due to its rarity, FDCS was often initially misdiagnosed. Identification or more of the following markers: CD21, CD23, CD35, podoplanin [6]., and CXCL-13 [7] by immunohistochemistry (IHC) can help differentiate FDCS.
There are few cases of retroperitoneal FDCS been reported in China, and none of the reported cases were manifested as Pelvic mass with low fever and a high level of WBC. In this study, we represented a case of FDCS which was primarily diagnosed as pelvic abscessa which was manifested as a big pelvic mass with low fever and weakness.
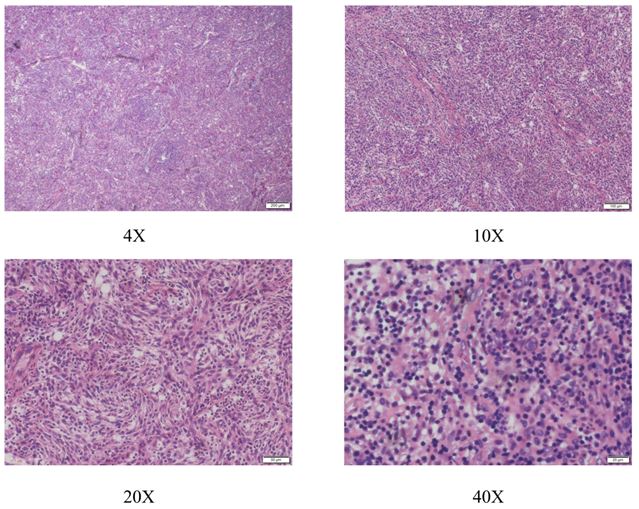
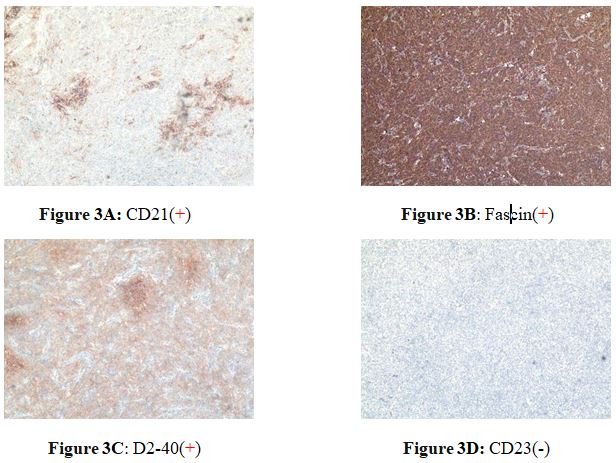
A 28-year-old woman suffering from a low fever and weakness since one week ago. After receiving antibiotic therapy from the local hospital, the syndrome seemed to turn better. However, one week later a big mass was found by ultrasound in the local hospital. At the same time, the laboratory tests showed the amount of WBC and CRP level were obviously increased, then she received a bone marrow puncture which revealed infectious bone marrow signs. She was admitted to Wuhan Union Hospital in 2020 May 4th. The pelvic examination showed that there was a round mass in the left pelvic with a clear edge and the mass was slightly moveable without tenderness. The CT scan revealed a cystic-solid mass (13*10*14cm) with lineal hyper-density shadow (Figure 1), which was indistinguishable with the left annex. The hyper-density shadow was calcification or hemorrhage inside. She received a laparoscopic abdominal exploration on May 13th. The tumor was removed and sent to the pathology center to have an intraoperative frozen biopsy. The tumor had a complete capsule, the contents were partly yellow gelatinous and partly necrotic. It was identified to a retroperitoneal tumor, but benign or malignant could not be distinguished in frozen biopsy. The appendix, Omentum and liver surface were examined intraoperatively. An abdominal drainage tube was placed. On the second day after surgery, the Blood test showed the WBC CPR decreased significantly after operation. On the fourth day after operation, the patient developed lymphedema in the perineum and left thigh. 170 ml of Pale-yellow fluid was drained from the drainage tube after that. Then the edema disappeared. The next day, 300 ml of fluid was drained. Then 200ml, 100ml and on the tenth day the drained fluid was 10ml. We removed the drained tube. The Blood test showed the WBC,Lymph CPR. Under HE staining, the tumor cells are spindle-shapedwith obvious nuclear atypia (Figure 2). Postoperative pathology confirmed that the tumor was a follicular dendritic cell sarcoma with immunohistochemistry showing D2-40(+) ,Fascin(+), CD21(+), CD23 (-), CD35(-), CD200(+), SSTR2(+), CD3(-), CD20(-), CD30(-), SMA(-), Desmin(-), S-100(-), CD34(-), PCK(-), ALK(-), EMA(-), Ki67(L1:10%), In-situ hybridization: EBER(-) (Figure 3A-D).
Etiopathogenesis of FDCS remains unclear. FDCS is often considered to be a low-to-intermediate grade sarcoma [8]. Monda, et al. first reported a case of intra-lymph node FDCS in 1986, and Chen et al. first reported a case of extra-nodal FDCS in 1994. The WHO defined the follicular dendritic cell tumors as a tumorous proliferation with FDC morphology and immunophenotype in 2001. The tumor is graded according to the cytological and clinical biological behavior [9]. Only about 200 cases have been reported so far.
Follicular dendritic cell (FDC) is mainly derived from the bone marrow and it is a member of the antigen-presenting cell (APC) family, which functions in the process of antigen recognizing, processing and presenting. Gene mutation or deletion may lead to the FDC over proliferation, and then results in the follicular dendritic cell sarcoma. FDCS mainly occurs intra or extra lymph nodes. The extra nodal FDCS may occur in tonsils, thyroid, palate, pharynx, liver, pancreas, peritoneum, breast, and lungs. FDCS most commonly occur in the head and the neck, while it is more common in young adults. FDCS usually presents as a slow, painless mass. No differences have been found between men and women.
The specific etiology and pathogenesis of FDCS are yet unclear. Several studies showed that FDCS may be related to the Epstein-Barr (EB) virus infection. While there are studies showing that no Epstein-Barr virus or EB virus latent membrane protein-1 was detected via in situ hybridization. It shows that the disease may not be directly related to EB virus infection. Except for liver and spleen, EBER RNA sequence cannot be detected in most FDCT cases located in other parts. The EB virus detection rate of FDCS is only 4%. there are studies reports that Epstein-Barr virus infection can be found in most liver follicular dendritic cell sarcomas, especially inflammatory pseudotumor-like follicular dendritic cell sarcoma. It is believed that the Epstein-Barr virus plays a key role in the occurrence of this tumor. While in this case, EBER was negative.
The diagnosis of FDCS is mainly based on the histological and immunohistochemical characteristics of the tumor cells, while other tissue cells and lymphoproliferative diseases need to be excluded. The clinical and imaging manifestations of FDCS are not specific. Most FDCS are well-boundaried, partially enveloped, and are nodular or lobulated; the cut surface is white or gray, with hemorrhage and necrosis in the tumor masses. Typical cell atypia composed of spindle-shaped or oval tumor cells and a large number of small lymphocytes can be found. Tumor cells are mostly arranged in flaky or interwoven strips, and sometimes striated structures can be seen. In some tumors, concentric vortex-like arrangements can be seen. The blood vessels of some tumors are more obvious. In a few cases, an expanded pseudovascular cavity can be seen, which is filled with eosinophilic red protein-like liquid, plus a sleeve-like structure formed by small lymphocytes that tend to gather around the blood vessels.
Under high magnification, the morphological characteristics of dendritic cells can be seen, showing as unclear cell boundaries, rich in cytoplasm and eosinophilic. The nucleus is oval or fusiform, the nuclear membrane is clear, the nucleolus is not obvious or small basophilic nucleoli, and pseudo-inclusion bodies are seen. There are lobular or multinuclear tumor cells. Coagulative necrosis can be seen in some tumors, which can be focal, flaky or map-like, and usually occurs in larger and deeper tumors. Very few cases may be mucus-like.
Immunological phenotype: specific follicular dendritic cell markers CD21, CD35, R4/23, Ki-FDC1p, Ki-M4 may be positive in FDCS, while epithelial markers CD18 and EMA, vascular markers CD34 And FVШ, hepatocyte markers Hepatocyte and AFP, and myogenic markers SMA are not expressed. Liver follicular dendritic cell sarcoma has a high positive rate of CD21 and CD35, but it is often weakly positive or focally positive. In 2002, the International Lymphoma Research Collaborative Group (ILSG) recommended the use of a group of antibodies against CD21, CD35, CD1a, S100, CD68, and lysozyme (LYS) based on the results of immunohistochemical analysis of 61 histiocytes and dendritic cell tumors as a routine Primary screening antibodies for histiocyte and dendritic cell tumors. FDCS is expressed as CD21(+), CD35(+), CD1a(-), S100(±), CD68(-), LYS(-) [9, 10].
Ultrastructure of FDCS: the cytoplasm of tumor cells is folded, rich in ribosomes, and lack of lysosomes or organelles. There are many complicated finger-like protrusions on the surface, which are interlaced and connected with adjacent cells. The protrusions are connected by desmosomes or desmosome-like structures, without Bireek particles and complex connections. The tumor cell nucleus is oval or fusiform, with chromatin concentrated on the edge of the nucleus, often with obvious nucleoli, irregular or wrinkled nucleoli, and small nucleoli can be seen. But there is no substrate and tension microwire [11].FDCS mostly exhibits inert biological behavior, with a low-to-intermediate grade of malignancy. Its biological behavior and prognosis are related to the patient’s age, tumor size, lymphoplasma cell infiltration, tumor cell mitosis count, necrosis, and lymph node metastasis [4, 5, 12]. At present, there is no standard for the treatment of FDCS. Surgical resection is the preferred treatment, but it tends to relapse after the surgery. Further surgically resection is preferred[4, 5]. Radiotherapy and chemotherapy are still uncertain. For patients with huge masses or surgery that cannot be cured, combined chemotherapy can be chose. At present, the most widely used chemotherapy is the combination of cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP) for the treatment of non-Hodgkin’s lymphoma, usually for 3 to 6 cycles. Other experimental programs include dexamethasone, high-dose cytarabine, cisplatin (DHAP), 2-chlorodeoxyadenosine (2-CDA), or concurrent radiotherapy. Those who cannot be completely resected, such as tumor cells with obvious atypia, mitotic figures> 5/10 HPF, large coagulative necrosis, high proliferation index, large tumor size (> 7 cm), and lack of adjuvant therapy, the prognosis is not Good.
In recent years, with the development of immunohistochemistry and electron microscopy technology and the deepening of understanding of FDCS, the diagnosis rate of FDCS has gradually increased. In this case, there were no specific clinical symptoms. Ultrasound and pelvic MRI can hardly differentiate diagnosis. The final diagnosis depends on the pathological diagnosis.