Persistent Sciatic Arteries Presenting with Acute Limb Ischaemia; A Report of Two Cases and Literature Review
Introduction: Persistent sciatic artery (PSA) is a rare anatomical anomaly which commonly presents with acute lower limb ischaemia among other possible modes of presentation.
Presentation: We report two cases of persistent sciatic arteries presenting with acute limb ischaemia and associated aneurysm formation which were successfully managed with surgery and endovascular interventions. A review of the literature with an analysis of potential risk factors is also reported as well as a proposed algorithm and guide to management options based on available literature. Conclusion: It is important to appreciate the existence of this rare anatomical variation and manage appropriately as it could be associated with various hazards.
Keywords: Sciatic artery; Limb ischaemia
The sciatic artery is initially the prominent vessel that supplies the lower extremity in fetal life but regresses as the femoral vessels take over. When present as a Persistent sciatic artery it may be the dominant arterial supply to the lower extremity and the femoral vessels are usually hypoplastic.
Patients with persistent sciatic arteries are prone to develop acute limb ischaemia and aneurysms of the vessel with its possible attendant complications [1]. Attempts at aspiration of gluteal masses and surgical procedures in the lower limb in a patient with undiagnosed PSA can also result in catastrophes hence, requiring a very high index of suspicion [2].
Persistent sciatic artery is a relatively rare condition with a reported incidence of 2.5 to 5 per 10,000 populations [3]. A systemic review of all published cases over a 43 year period between 1964 and 2007 by Hooft, et al. revealed only 159 PSAs in 122 patients [4].
A 65 year old female of Nigerian origin presented with sudden onset pain and numbness of her right leg and foot, coldness of the foot and inability to walk.
She had previously experienced a painful toe and leg swelling in the preceding year without a clear diagnosis. She was a known hypertensive on treatment for over 30 years. She had never smoked and was not aware of any gluteal swelling prior to presentation. Initial evaluation of her right leg and foot revealed pallor, reduced skin temperature, reduced sensation and paralysis of the toes. Her femoral pulses were markedly reduced bilaterally. The popliteal pulses were normal bilaterally. Her right posterior tibial and dorsal is pedis pulses were absent. The left dorsalis pedis and posterior tibial pulses were present.
She had a BMI of 22.4, total cholesterol 6.25 mmol/l (mildly elevated), triglycerides 0.69 mmol/l, HDL 1.32 mmol/l, LDL 4.62 (mildly elevated).
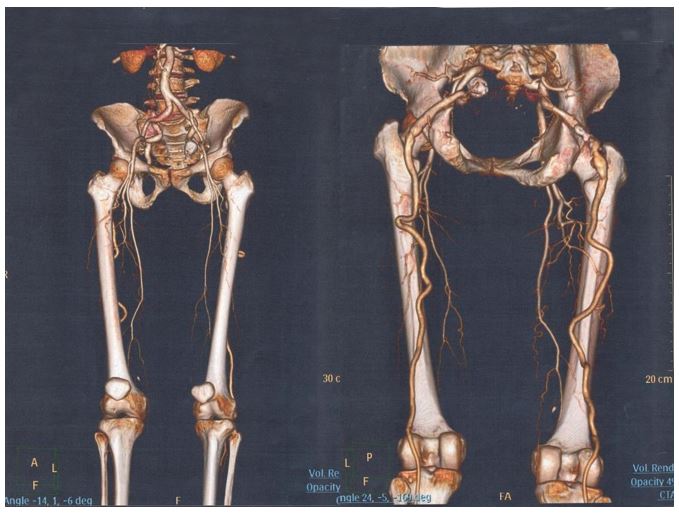
A Computerized Tomography angiogram (CT-angiogram) revealed hypoplastic femoral arteries with branches terminating in the thighs bilaterally, bilateral persistent sciatic arteries continuing to form the popliteal arteries and bilateral fusiform aneurysmal dilatation of the sciatic arteries in the gluteal areas. It was also noted the popliteal arteries were more lateral behind the lateral femoral condyles than would be found in normal anatomy (Figure 1).
An impression of acute limb ischaemia due to thromboembolism complicating aneurysmal persistent sciatic artery was made and treated by thrombo-embolectomy using a Fogarty catheter through a popliteal artery approach. A posterior skin crease incision was used due to the unusual lateral and superficial position of the popliteal artery. She was discharged a week after the procedure and at follow up 4 months later she was ambulant but had some residual numbness of the foot and occasional pain but no claudication.
Ten months later, at another institution, she underwent a right femoral-popliteal bypass grafting using reversed autologous vein and coil embolisation of the right sciatic artery aneurysm.
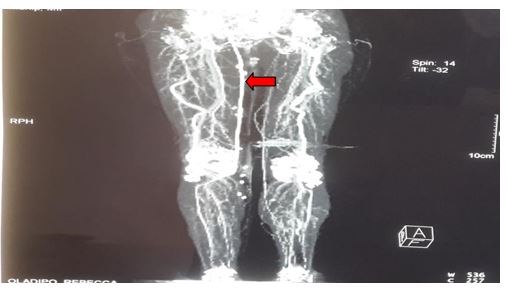
Follow up post procedure CT angiograms at 5 months confirms a patent graft and no filling of the sciatic aneurysm (Figure 2). Currently, she has no symptoms but continues to be on follow up for the left PSA and associated aneurysm.
A 59 year old female presented with a one day history of sudden onset severe right foot pain associated with numbness and tingling sensation. She also reported having experienced pain and swelling of the same limb as well as being aware of a painless gluteal swelling on the same side for over 2 years. She had been repeatedly treated at other medical facilities without a clear diagnosis. She had also been on treatment for hypertension for 2 years.
Examination of the right lower limb revealed gangrene of the toes with leg oedema, tenderness and blister formation. The foot was cold and insensate up to the ankle joint. The ipsilateral femoral pulse was markedly diminished but popliteal pulse felt normal with absent dorsalis pedis pulse. A pulsatile swelling with ill-defined margins was found on the ipsilateral gluteal region.
The CT angiogram done then reported it to be a right superior gluteal artery aneurysm. She underwent a below knee amputation as an emergency.
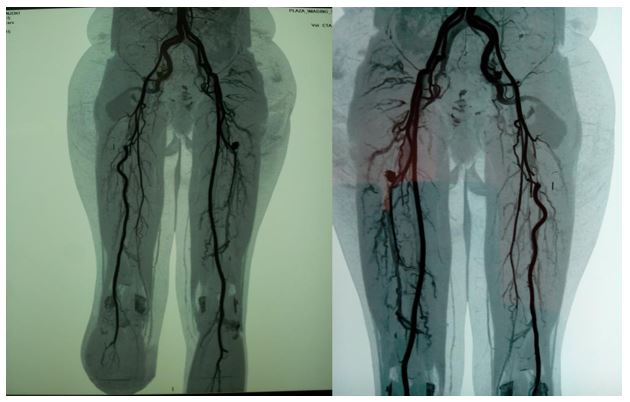
On further post-operative review the possibility of a PSA was entertained. A repeat CT Angiogram (Figure 3) revealed a large right internal iliac artery emerging out of the sciatic notch to form a complete PSA with a 5.4 by 7.6cm fusiform aneurysmal dilatation with an intramural thrombus and continuing to form the popliteal artery. The femoral artery was hypoplastic and terminated as thigh muscular branches.
The patient subsequently underwent aneurysm management by ligation of the internal iliac artery as the femoral artery branches were deemed adequate for the already amputated limb.
Persistent sciatic artery was first described and reported in the Lancet in 1832 by Green [5]. In normal developmental anatomy the sciatic artery is initially a prominent vessel for the lower limb in early fetal life but regresses as the femoral vessels take over. The incidence of PSA is quoted to be between 2.5 to 6 per 10,000 population in most studies [3,4,6,7]. No gender has been shown to be more affected5 although several reports have suggested a slight female preponderance [4,8,9]. Despite its developmental origin, the clinical presentation usually occurs in mid life and in the elderly. Mean age at presentation in most reports is in the sixth decade with a range from 15 to 85 years [4,5,7,8,9]. An exceptional case diagnosed at autopsy of a 32 week old fetus has also been reported [4].
Different rates of occurrence of bilateral PSA (as seen in our first case) are reported in the literature. Marincola, et al. reported 12% [10]. Ikezawa gives a rate of 22% although earlier report by Mayschak reported even a higher incidence of 50% [5,9]. A recent review of the literature by Hoft, et al. of 122 patients found bilateral case in 30% [4]. In cases of unilateral PSA, either limb may be affected in equal proportion [4].
Persistent sciatic arteries have been classified as complete or incomplete as originally proposed by Bower, et al. [11]. When the persistent sciatic artery is complete, it provides the major blood flow to the lower extremity and continues to form the popliteal artery. The superficial femoral artery will be hypoplastic and has no direct continuity with the popliteal artery except through collaterals. The complete type occurs in about 63-68.5% of cases [4,9,12]. In the incomplete type, the persistent sciatic artery is present but is hypoplastic and terminates in the thigh. The superficial femoral artery is the main supply distally through the popliteal artery.
Others have suggested a more comprehensive classification of PSA. Pillet, et al. described four types of PSA which was later modified by Gauffre, et al, by adding a fifth class [4,13-15] (Table 1).
However, for purposes of patient management most clinicians prefer to use the original simple classification of PSA as either being complete or incomplete [9-11,16]. Our first patient had bilateral complete PSA (Bower classification) or bilateral type IIa according to the Pillet-Guaffre classification. The second patient had unilateral complete PSA.
When the sciatic artery is complete and forms the popliteal artery the latter may be more lateral and superficial in position than is usually the case, a finding which was reported by Hutchinson in 1967 and has only been rarely mentioned by others since [17,18]. Our first patients had both popliteal arteries being located behind the lateral femoral condyles and were more superficial than is usually the case. It appears a PSA may be associated with abnormal popliteal artery anatomy which may have further implications in case of surgical intervention in such patients.
Symptomatic PSA may present with aneurysm related symptoms (pulsatile gluteal mass, distal ischaemia due to thrombosis or embolism from a thrombosed aneurysm, sciatic pain due to compression of the nerve by the aneurysm or rarely aneurysm rupture) [12].
Patients with PSA can be discovered when they present clinically with the complications or diagnosis may be incidental on evaluation for other reasons. In some cases unusual bleeding or abnormal anatomy at surgery has led to the diagnosis [6,19]. Some have been found during postmortem dissections [4,20-22].
The diagnosis of PSA may be suspected when a patient has weak or absent femoral pulse with a normal or strong popliteal pulse as was seen in our second patient(Cowie’s sign), a clinical scenario that is easily missed by most clinicians due to lack of awareness.
Patients with PSA have an aneurysm development rate of 41-48% [4,9,11,18,23]. The aneurysm most commonly affects the part of the PSA behind the greater trochanter under the gluteus maximus. Many reasons have been put forward to possibly explain the high incidence of aneurysmal change; repeated trauma in view of the anatomical site of the vessel has been the most mentioned. Others have however postulated a possibility of a primary abnormality in the development of the persistent sciatic artery with less elastic tissue in its walls rendering it vulnerable to aneurysm formation [4,10,16,24]. Most of the aneurysms are fusiform and frequently have a mural thrombus [9]. PSA aneurysms have a high incidence of thromboembolic complications among other symptoms which may occur due to pressure on the sciatic nerves, or rupture of the aneurysm [16,18]. The two patients presented here had aneurysms of the PSAs with thrombo-embolic complications.
Limb ischaemia may occur in patients with PSA due to thrombosis of an aneurysm, embolism from a mural thrombus in the aneurysm or thrombosis of a non-aneurismal PSA. This could be as a result of multiple factors associated with PSA such as atherosclerotic changes, tortuosity of the PSA, sluggish flow and repeated trauma due to its position.
Our first patient presented with acute limb threatening ischaemia due to thromboembolism from the mural thrombus in the aneurysm while the second developed foot gangrene.
An aneurysmal PSA may result in sciatic nerve symptoms (pain, numbness or muscle weakness) due to pressure on the sciatic nerve [1,5]. Sometimes this may be the presenting feature.
Neurological complications may also occur following open surgery for a PSA aneurysm due to the close association and increased risk of sciatic nerve injury [25]. Our first patient continues to Experience some pain and numbness of the limb without claudication which could be attributed to sciatic nerve compression.
Computerized Tomographic Angiography (CTA) has become the most useful tool in many centers for diagnosis and evaluation of PSA. We suspect many more PSA’s are being identified in recent times due to CTA than was previously the case. The incidence of PSA may even be higher than previously quoted. Jung, et al. have noted a higher incidence of 1.63% in their series since adopting routine use of CTA for evaluating lower extremity arterial disease [26]. CTA can also define the aneurysm formation and describe the degree of intraluminal thrombosis [26].
Conventional angiography may miss out some cases of PSA if the contrast is injected more distally at the external iliac artery level leading to erroneous diagnosis of superficial femoral artery occlusion [26].
In a review by Hoft, et al, angiography was the most used diagnostic tool [4]. This is because the study was a review covering cases even before the era of CTA when conventional angiography was the main investigation for arterial disease.
Magnetic resonance angiography (MRA) has also been recommended by others as a possible better alternative for evaluation of PSA as it does not involve use of contrast [19,22]. Duplex ultrasonography may also be useful but will not add any value where CTA or MRA are available. Conventional or Digital Subtraction Angiography (DSA) may be reserved for cases where some endovascular intervention is planned.
The management of individual cases of PSA is often multimodal and tailored to patient symptoms, complications present or anatomical class of the PSA.
The anatomical classification of a PSA is a useful tool in outlining management guidelines. The goals of treatment are largely to prevent limb ischaemia or restore circulation, treat aneurysm when present and prevent sciatic nerve injury during treatment.
A patient with an asymptomatic PSA without an aneurysm does not need intervention but should be followed up with regular clinical assessments, ABPI measurements and duplex scans to detect aneurysm formation or occurrence of ischemic symptoms [4,12,16,27].
In some cases a PSA without an aneurysm may present with ischemic symptoms since these vessels have a tendency to early atherosclerotic changes, stenosis, tortuosity and sluggish flow making them vulnerable to thrombotic episodes [4,9,23]. This usually occurs with a complete PSA where it is the main supply to the extremity. Such cases can be managed by open surgical procedures or endovascular interventions. Gabelman, et al. successfully managed a non- aneurysmal stenotic complete PSA with endovascular stenting [23]. However there is still the fear of complications or recurrence of ischaemia especially due to pressure on the stent as the patient
Sits on the stented area. A case of symptomatic PSA without an aneurysm presenting with distal thromboembolism has also been managed successfully by thrombo-embolectomy alone [28]. This can be a management option but the risk of recurrence still remains and patient follow up is advised.
Many have advocated a bypass procedure with exclusion of the PSA and thrombo-embolectomy of the popliteal artery [9]. The bypass can be anatomical or extra-anatomical. The graft material used can be autologous saphenous vein or a synthetic material. Femoro-popliteal reversed saphenous vein graft has been most used. However if the femoral artery is significantly hypoplastic the inflow can be from the iliac vessels [9].
Some form of intervention is recommended by many for asymptomatic PSA with associated aneurysm with the aim of avoiding thromboembolic ischemic complications, reducing risk of rupture and relieving pressure on the sciatic nerve although others still favour conservative approach of follow up and monitoring [12]. Our first patient is on conservative follow up for her asymptomatic left PSA aneurysm.
Management of aneurysmal PSA with symptoms depends on whether the PSA is complete or incomplete and any complication(s) present. An incomplete PSA aneurysm can be managed by aneurysm ligation, resection, or endovascular exclusion by embolization [4,12,29].
When the PSA is complete, management of the aneurysm should also include a revascularization procedure to avoid possible distal ischaemia. This can be via an in situ interposition graft or exclusion bypass procedures as described earlier for non-aneurismal PSA or endovascular stenting4. In endovascular procedures, a stent can be used to exclude the aneurysm at the same time maintaining patency of the PSA. This has the advantage of treating the aneurysm at the same time and avoiding distal ischaemia in one procedure. However, as already pointed earlier when an interposition graft or stent is used there is risk of occlusion due to repeated trauma in the sitting position and the PSA will still be susceptible to atherosclerotic changes [4,9]. Endovascular procedures have an additional advantage since the risk of sciatic nerve injury is avoided that could occur in open aneurysm repair [4].
The most favoured approach for a symptomatic complete PSA with associated aneurysm in most recent reports is autologous vein femoral popliteal bypass and aneurysm occlusion by embolisation as was the case for our first patient [30].
It is important to appreciate the existence of this rare anatomical variation in considering the differential diagnosis of lower limb ischaemia, gluteal masses, and sciatic nerve symptoms. Awareness of its existence is equally important to avoid dangerous diagnostic needle aspirations for gluteal masses and surgical misadventures that may occur while operating in the lower limb for other reasons in such patients.
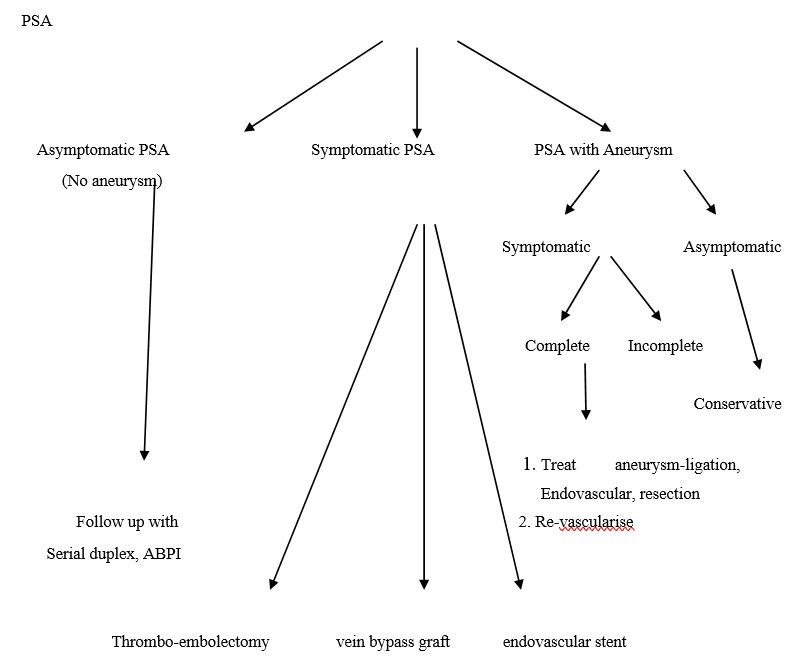
We have made an attempt to propose an algorithm and guide to management options based on available literature (Figure 4).
We would like to acknowledge Mr. Mark McCarthy and his colleagues at University Hospitals of Leicester, Department of Vascular and Endovascular Surgery who performed the coil embolization of the aneurysm and saphenous bypass graft on our first patient for furnishing us with the details of the procedure.