Phytochemical Composition of Calyx Extract of Roselle (Hibiscus sabdariffa L.) Genotypes
Thirty five Roselle genotypes were evaluated at the experimental field of Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706 during August 2015 to February 2016. Fifteen genotypes were selected for the analysis of phytochemical composition and the nutritional quality of calyx extract. The objective of the research was to estimate phytochemical constituents such as carotenoid, flavonoid, phenol, anthocyanin, and vitamin C content and antioxidant activity of calyx extract of Roselle. The genotype BUM-003 (873.61 μg/g) contained high amount of phenol among the 15 genotypes followed by BUM-007 (867.84 μg/g) and the lowest (481.57 μg/g) in genotype 4561. The high amount of carotenoid was estimated in the genotypes BUM-003, BUM-004, BUM-007 (30.18, 30.95, 29.69 μg/g, respectively). The genotypes 1740 (7.07 μg/g) and 4561 (7.73 μg/g) contained less amount of carotenoid. All the genotypes contained high amount of anthocyanin (>80 μg/g) except 1740, 4561, BUM-002. High amount of flavonoid was estimated in the calyx extract of the genotypes BUM-003 (399.15 μg/g) and BUM-004 (407.34 μg/g). The highest amount of antioxidant was estimated in the genotype BUM-002 (492.87 μg/g) followed by 4385 (488.09 μg/g), 4920 (487.99 μg/g), 4561 (476.86 μg/g), 4601 (473.10 μg/g), BUM-003 (469.44 μg/g), BUM-004 (463.59 μg/g) and BUM-007 (461.80 μg/g). Antioxidant activity was determined by 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) radical scavenging assay and maximum was found in the genotype 4920 (95.09%) followed by 4385 (94.12%), BUM-002 (93.55%) and minimum (72.83%) in the genotype 4537. The highest amount of vitamin C was estimated in the genotypes BUM-003 (312.97 μg/g) and the lowest in the genotype 4561 (26.20 μg/g). From the overall phytochemical composition it might be concluded that the calyces of the genotypes 4385, 4920, BUM-002, BUM-003, BUM-004, and BUM-007 are the excellent source of natural antioxidants.
Keywords: Roselle (Hibiscus sabdariffa L.); Nutrition; Chemical Composition; Calyx ExtractISSN:
Roselle (Hibiscus sabdariffa var sabdariffa L.) is a tropical tetraploid (2n=72) annual plant species in Malvaceae family, which originated from West Africa [1]. The calyx is the most important part of the plant. It contains the valuable components which determine the quality of the product namely: colour (anthocyanins), flavour (organic acid) and aroma. It has natural and considerably unique nutritional characteristics, in particular its high contents of vitamin C and anthocyanins. Roselle has some medicinal properties. It contains high levels of antioxidants which reduce body fat, assist in weight loss, reduce high blood pressure and blood sugar, and help in digestion [2,3]. Roselle is also used in traditional medicine. It was proved by many experiments Roselle shows antiypersensitive, antihyperlipidimic, hepatoprotective, diuretic, anticancer, antioxidant and many other properties [3,4-6]. In Bangladesh, it grows first for its fleshy calyces, leaves and the second for its phloem fiber at homestead level. Many countries in the world give importance on Roselle cultivation. China and Thailand is the largest producers in Asia [7]. Despite of its potential economic importance, it has received little attention and information regarding, genetics, breeding and production. In Bangladesh, it grows first for its fleshy calyces, leaves and the second for its phloem fiber at homestead level. The fleshy calyces are the most popular and are used for making drinks, jam, jelly, syrup, gelatin, pudding, cakes, ice cream and flavors and dried and brewed into tea, among other things [8]. Roselle is an indigenous crop in Bangladesh. In Bangladesh, there is no high yielding varieties of Roselle due to no attempt has been made for genetic improvement of this crop. The yield of calyx is very low in farmer’s conditions in Bangladesh due to the poor potential of cultivated varieties. In order to improve the yield of Roselle, plant breeders should have information on the nutritional quality of genetic materials for the development of high yielding variety with quality traits. For this purposes, 35 available genotypes of Roselle collected from local and exotic sources were evaluated to know the nutritional quality of identified Roselle genotypes for industrial use.
Thirty five Roselle genotypes were studied for their agronomic performance, genetic variability, and character association of 15 quantitative traits [days to first flowering, days to 100% flowering, days to first fruit setting, days to first edible maturity, branches per plant (no.), plant height (cm), internodal length (cm), length of fruit (mm), width of fruit (mm), fruit weight (g), calyx weight (g), fruits per plant (no.), calyx fruit ratio, calyx ball ratio and calyx yield (g/plant)] at the experimental field of Department of Genetics and Plant Breeding (GPB), Bangabandhu Sheikh Mujibur Rahman Agricultural University (BSMRAU), Gazipur 1706. Based on agronomic performance (fruits per plant>250, individual calyx weight>500g and calyx yield>800g/plant) of 35 genotypes, 15 genotypes were selected for the analysis of phytochemical composition and the nutritional quality of calyx extracts. Phytochemical analysis was done at the physiology laboratory of the department of Crop Botany, BSMRAU, Gazipur 1706, during the period from August 2015 to February 2016.
Total carotenoid content was determined according to the procedure of Lachman, et al. [9] with slide modification. Total carotenoid content was expressed as micrograms of lutein equivalent per gram of flesh weight sample (μg LE g-1 FW) ± SE for three replications from the lutein standard curve.
Anthocyanin content was determined with slight modifications as described by Hughes and Smith [10]. For preparing the extraction solution 7ml 6M HCl was added into a volumetric flask where 70 ml methanol and 23 ml of distilled water were taken earlier. Briefly, 1 g fresh sample was taken into an ice cold vial and 5 ml extraction solution was added here. After keeping the vial at 4 °C for 24 hours 2 ml of the solution was centrifuged with 2 ml distilled water and 2 ml chloroform. The aliquot was taken into a cuvette and absorbance was measured at 530 nm. The anthocyanin content was expressed as micrograms of cynadin-3-glucoside equivalent per gram of fresh potato sample.
The extraction was done by following the procedure of Nayak, et al. [11]. Briefly, one gram of peeled, chopped Roselle calyces was homogenized with 10 ml of HPLC grade methanol to a uniform consistency by mortar and pestle. The samples were centrifuged at 30,000× g at 4 °C for 20 min and the supernatants stored at -20 °C for further analysis.
For the determination of DPPH scavenging percentage 1 ml plant extract and standard were taken into a test tube. After adding 3 ml of 0.2 mM of DPPH solution into the test tubes, they were incubated for 5 min at 25 °C and finally the absorbance was measured at 517 nm. The antioxidant activity was expressed as DPPH scavenging percentage (%).
The content of total phenolic compounds was determined spectrophotometrically according to the Folin-Ciocalteu method [12,13] with slight modification. The absorbance of reaction solutions was measured at 765 nm against a blank sample. The measurements were compared to a standard curve of Gallic acid solutions and expressed as micrograms of Gallic acid equivalents per gram fresh weight ± standard deviation (μg GAE g-1 FW ± SE).
Total flavonoid content was determined according to the procedure of Zhuang, et al. [14] with slide modification. Total flavonoid content was expressed as micrograms of quarcetin equivalent per gram of flesh weight sample (μg QE g-1 FW) ± SE for three replications from the quarcetin standard curve.
From 1 g of Roselle sample AsA was extracted by using 4% TCA (Trichloro Acetic Acid) and the volume was made upto 10 ml with the same. After centrifugation (2000 rpm, 10 min) the supernatant of the Roselle extract was treated with a pinch of activated charcoal and kept for 5 min after shaking. Charcoal particles were removed by centrifugation and aliquots were used for AsA estimation. 1 ml of extract was added with 4% TCA, DNPH (Di-nitrophenyl-hydrazine) reagent and thiourea by following the method of Kapur, et al. [15]. After incubation (37 °C, 3 hrs), 85% sulphuric acid was added to the cooled sample. Absorbance was read at 540 nm against blank.
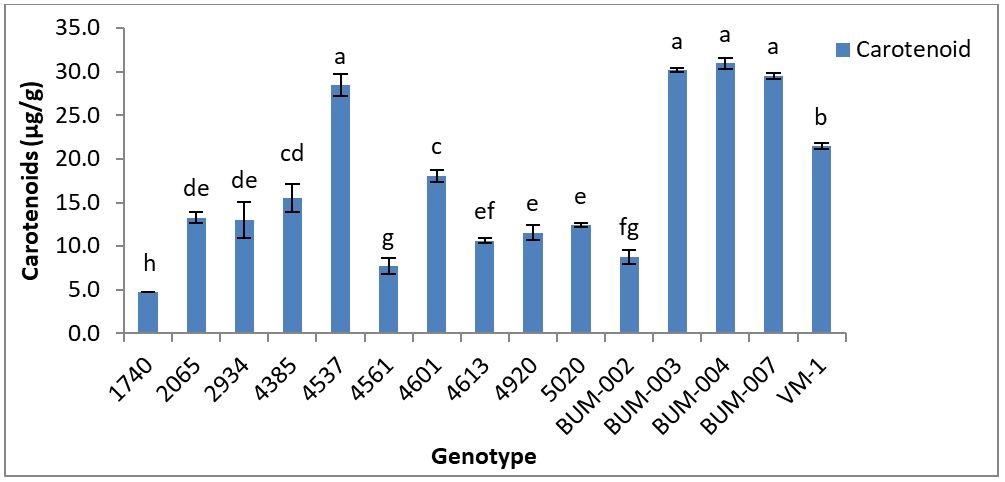
Carotenoid have an immense value of human health and many people are seeking ways to incorporate these health-brimming compounds into their diets. Carotenoid supports eye health, cardiovascular health [16]. In this finding, the range of carotenoids was 31-4.8 μg/g. The genotypes BUM-004 (31 μg/g) contains the highest amount of carotenoids followed by BUM-003 (30.2 μg/g), BUM-007 (29.5μg/g) which are statistically same. The genotypes 1740 (4.8 μg/g) contains the lowest amount of carotenoids followed by genotypes 4561 (7.7 μg/g), BUM-002 (8.8 μg/g) (Figure 1). Peng‐Kong Wong, et al. [17] reported the amount of β‐carotene contents in Roselle extract was 1.88mg/100g.
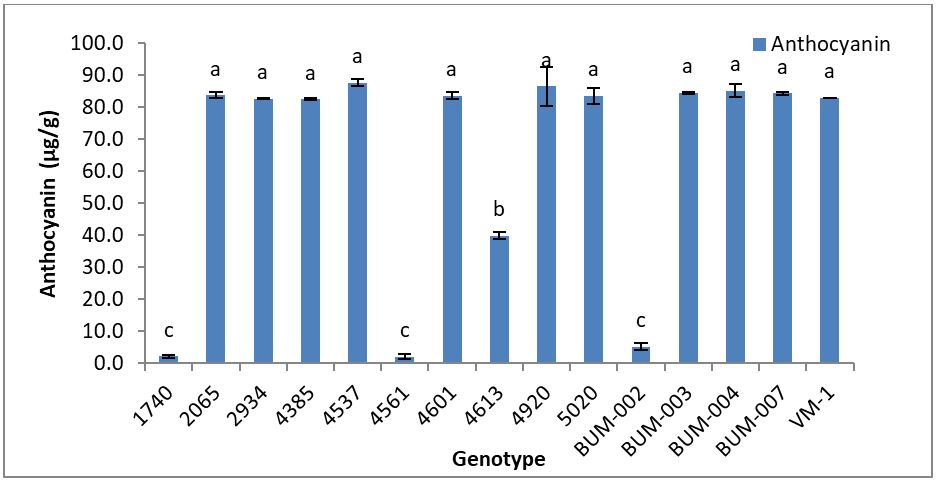
Anthocyanin may help night vision and improve overall visual acuity. Anthocyanin may decrease cholesterol levels, lower blood pressure, increase blood flow, enhance capillary strength and fight oxidative stress [18,19]. Total anthocyanin ranged from 2.1-87.7 μg/g (Table 1). The genotypes 4537 (87.7 μg/g) contained the highest amount of anthocyanin followed by genotypes 4920 (86.5 μg/g), BUM-004 (85.2 μg/g) which are statistically similar. The genotype 4561 (2.1 μg/g) contained the lowest amount of anthocyanins followed by 1740 (2.2 μg/g), BUM-002 (5.2 μg/g) which are statistically similar (Figure 2). The calyx of Hibiscus was rich in anthocyanins [20].
Juliani, et al. [21] found the total anthocyanin content of 10-15μg /g (DM) in the Vimto variety of different origins and years of production. The difference between the values of present study and the literature is probably due to extraction method (extraction solvent, time and temperature) which was not the same as well as methods of analysis used. The composition and total content of the anthocyanins in Hibiscus can also be mainly affected by cultivar, year of production, soil and climate conditions.
Flavonoids inhibit a variety of Cancers in animals. High flavonoid intake can reduce human cancer risk [22] Total flavonoid contents ranged from 9.31-404.40 μg/g. Among the fifteen genotype, the highest flavonoid content found in genotype 4537 (404.40 μg/g) followed by BUM-004 (402.90 μg/g) which are statistically similar. The lowest flavonoid content found in genotype BUM-002 (9.31μg/g) followed by 4561 (9.32 μg/g), 1740 (9.36 μg/g) which are statistically similar (Table 1).
Total phenol content was found to be varied from 488.98-869.45 μg/g FW of Roselle calyces. The genotype BUM-003 (869.45 μg/g) contained the highest amount of phenol content followed by genotype BUM-007 (867.84 μg/g), 2065 (526.51 μg/g) which were statistically different from each other. The genotype 4561 (488.98 μg/g) contained the lowest amount of phenol content followed by 4385 (494.16 μg/g), 4613 (494.22 μg/g) which were statistically different (Table 1). Kilima, et al. [23] found that the total phenolic content of Roselle extracts ranged from 108 to 546 μg/g. Luvonga, et al. [24] reported that the total phenolic content of Roselle extracts ranged from 582 to 606 μg/g. Studies demonstrated that the calyx of Hibiscus was rich in phenolic compounds [20].
The health benefits of vitamin C or Ascorbic acid are many. It prevents the scurvy, treatment of common cold, boosting the immune system, lowering the hyper tension, treatment of cancer; maintain elasticity of the skin [25]. The human body doesn’t have the capacity to generate vitamin C. The highest amount of ascorbic acid found in genotype BUM-004 (424.19 μg/g) followed by BUM-003 (321.35 μg/g), BUM-007 (200.30 μg/g) which were statistically different. The lowest amount of ascorbic acid found in genotype 4561 (26.20 μg/g) followed by BUM-002 (41.35 μg/g), 1740 (47 μg/g) (Table 1). Babalola, et al. [26] found in his findings that the green Roselle calyces contained 865 μg/g ascorbic acid. Kilima, et al. [23] found that the Roselle calyces contained 400-865 μg/g ascorbic acid. Mohamad, et al. [27] described that the Roselle variety Terengganu contains 234 μg/g ascorbic acid.
Increasing one’s antioxidant intake is essential for optimum health, especially in today’s polluted world. Antioxidant intake can protect body against heart problem, eye problems, memory problems, mood disorders, immune systems problems [28]. Total antioxidant ranged from 265.66-485.85 μg/g BHT eqv. The genotype BUM-004 (485.85 μg/g BHT eqv) contained the highest amount of antioxidant followed by BUM-003 (481.49 μg/g BHT eqv), BUM-007(481.03 μg/g BHT eqv) which were statistically similar. The genotype 1740 (265.66 μg/g BHT eqv) contained the lowest amount of antioxidant followed by 4561(265.67 μg/g BHT eqv), BUM-002(266.5 μg/g BHT eqv) which were statistically similar (Table 1). Luvonga, et al. [24] reported that the range of total antioxidant of Roselle was 230.01 to 235.34 μg/g.
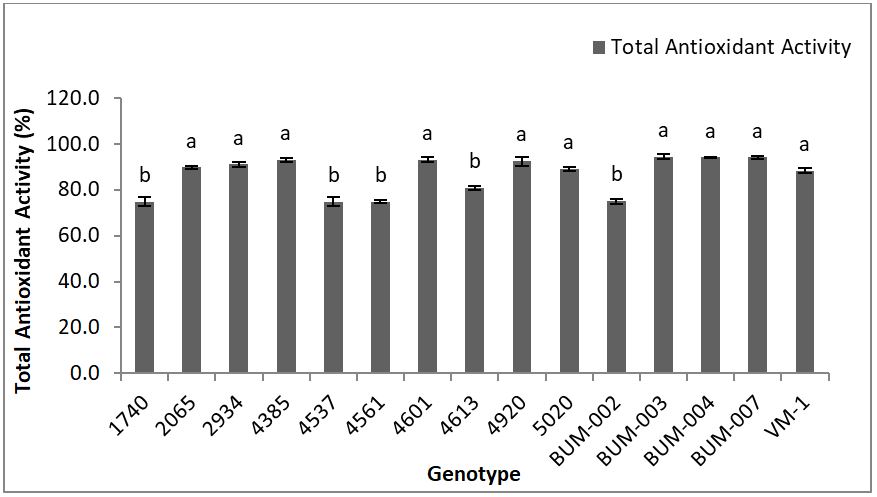
The highest antioxidant activity was found in the genotype BUM-004 (94.50%) followed by BUM -003 (94.24), BUM-007 (94.22%), 4601 (93.23%), 4385 (92.98%), 4920 (92.59%) which were statistically similar. The lowest antioxidant activity was found in the genotype 1740 (74.77%) followed by 4537 (74.77%), 4561 (74.78), BUM-002 (75.1%) which were statistically similar (Figure 3). Obouayeba, et al. [29] found the highest antioxidant activity (97%) in Roselle extracts.
The calyx extract of the genotype BUM-004 contained the highest amount of carotenoids, ascorbic acid, antioxidant and antioxidant activity among the 15 genotypes selected for chemical analysis based on agronomic performance. The genotype 4537 contained the highest amount of anthocyanin and flavonoid. The genotype BUM-003 contained the highest amount of phenol in the calyx extract.
The present project work is financed by the Research Management Committee (RMC) of Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706, Bangladesh. The authors would like to acknowledge their gratitude towards university authority for financial support
The author(s) declare that there is no conflict of interest regarding the publication of this paper