Prevalence, Incidence and Characterization of Neurocognitive Impairment in Acute Coronary Syndrome. A Systematic Review and Meta-Analysis.
Background: Neuropsychological research in cardiovascular diseases has focused mainly in heart failure. The present systematic review aims to determine the incidence of neurocognitive dysfunction in acute coronary syndrome (ACS) and to define the neurocognitive functions most affected in ACS.
Methods: The systematic review was performed in June 2016 on Pubmed. No restrictions were established regarding publication date, language, age of the participants in each study, nor type of study or experimental design.
Results: Four studies were selected out of 74, once they address to at least one of the aims. The prevalence of neurocognitive dysfunction ranges between 10.51% and 66.8%. We could determinate the incidence rate in two studies, ranging from 0.0277 cases per year to 0.416 cases per month. Alterations in verbal memory, language and executive functioning were found.
Conclusions: The variability of prevalence rates of neurocognitive dysfunction in ACS across studies may be due to the use of different neuropsychological instruments, different times of neuropsychological assessment and diverse demographical and clinical characteristics of the samples. In the future, further studies must clarify the neurocognitive tests that are more sensitive to ACS. The study of the interaction between risk factors, biomarkers, behavioral and environmental aspects as well as the clinical features of ACS and its treatment on brain and neurocognitive functioning, should also be clarified.
Keywords: Cardiovascular diseases; Incidence; Neuropsychological assessment; Cognitive impairment
Cardiovascular diseases (CD) are the main cause of mortality and morbidity in Europe [1,2]. The acute coronary syndrome (ACS) is the most prevalent CD in developed countries, for both genders [3]. ACS is the result of the rupture or erosion of the atherosclerotic plaque, with several degrees of thrombosis and distal embolization [4].
Several studies have pointed to a prior history of ACS in dementia patients [5]. In fact, it has been established that ACS patients have a five times greater risk of developing any form of dementia [6]. This observation relates to the increased risk of cerebrovascular insults, such as ischemic stroke and transient ischemic attack, in the context of coronary disease and also implies a vascular basis for neurodegenerative conditions such as Alzheimer’s disease [7].
Prospective studies point to the relation between several cardiovascular diseases such as ACS and lower performance on neurocognitive screen measures [8]. Neuropsychological research on CD has focused mainly in heart failure, a common denominator to several cardiac pathologies including ACS. Deficits in executive functioning, reduction in simple attention tasks, psychomotor speed, immediate memory and mental processing speed have been reported [9,10]. At six years follow-up of these patients showed a mild but significant decline of visual memory, visuoconstructive ability, verbal fluency, executive and global cognitive functioning [11]. However, the inclusion of heart failure patients with other CD diseases other than ACS, makes it difficult to determine the incidence and the characterization of neurocognitive deficits.
In this context, the present systematic review aims to answer to two questions: What are the prevalence and incidence rates of neurocognitive dysfunction in ACS?; What are the neurocognitive functions most affected in ACS?
The systematic review was performed in June 2016 based on Pubmed (US National Library of Medicine National Institutes of Health). No restrictions were established regarding publication date (since the beginning to June 2016), language, age of the participants in each study, nor type of study or experimental design. We used the following search terms: [acute coronary syndrome* AND (neurocognition OR cognition OR neuropsychological OR neuropsychology OR “cognitive impairment” OR dementia)].
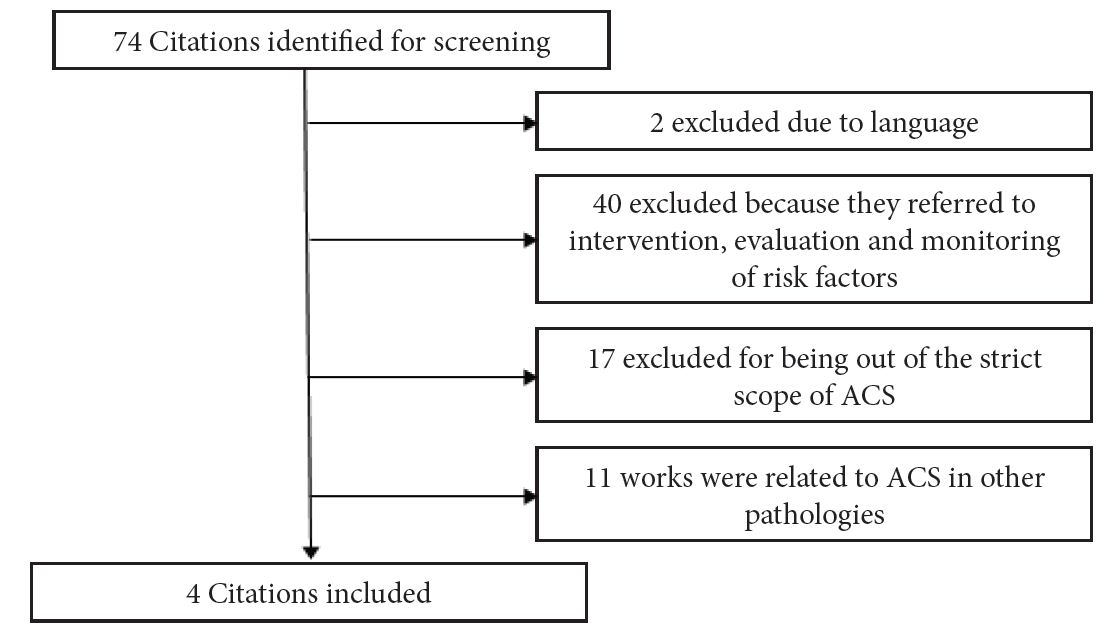
The search generated 74 papers (original= 52; review= 18; clinical cases= 4). 40 were excluded because they referred to intervention, evaluation and monitoring of risk factors (e.g. Diabetes, hypertension, depression, obesity) and mortality in ACS. 17 papers were excluded for being out of the strict scope of ACS (e.g. Stroke, heart failure). 11 works were related to ACS in other pathologies (e.g. Cancer, dementia) and therefore were also excluded (Figure 1). 2 works were excluded due to the fact that none of the authors of this review were sufficiently proficient in the language of those papers. One was written in Japanese and other in Norwegian. Only four papers were selected due to the fact that they provide answer at least to one of the formulated questions (Table 1). For each work prevalence was determined by using the formula:
(Number of current cases (new and preexisting) at a specified point in time/Population at the same specified point in time)× 100
Incidence rate was determined in two studies through the following formula:
Number of new cases of disease during specified time interval/ Summed person years of observation or average population during time interval.
The present review aimed to determine the prevalence and incidence of neurocognitive dysfunction and to identify the main neurocognitive deficits in ACS.
The prevalence of neurocognitive dysfunction ranges between 10.51% and 66.8%. The study reporting higher prevalence level [12], classified 52% of participants as having mild cognitive disorder and 14.8% having dementia. The majority of the participants in that study had a prior history of coronary heart disease and a high degree of comorbidities, such as diabetes, chronic kidney disease and depression. The authors have found an association between cognitive dysfunction and medication nonadherence, especially in patients with mild cognitive disorder [12]. In this study, the time elapsed since the ACS and neuropsychological assessment is not clear.
The study of Mixon and colleagues [13], assessed patients shortly after admission. 8% of the participants received a diagnosis of both ACS and acute decompensated heart failure. This study found an association between worse cognitive dysfunction and misunderstanding in frequency of medication [13]. Considering ACS patients only, the prevalence of neurocognitive dysfunction is 10.52%.
The study of Volonghi and colleagues [14], points to higher rates of neurocognitive dysfunction at 1 year and 5 years after ACS in comparison to transient ischemic attack (TIA) and minor stroke patients. 9% of ACS patients presented neurocognitive dysfunction at 1 year and did worse on Mini Mental Sate Examination (MMSE) when compared to TIA and minor stroke patients. Five years after the ACS, 10% of the patients obtained scores below the cut-off on MMSE and 63% showed deficitary results on Montreal Cognitive Assessment (MoCA). At this period, ACS patients general level of neurocognitive functioning was similar to those with TIA or minor stroke [14]. During the five year period the common denominator of neuropsychological assessment was the use MMSE. Thus, taking that into account, the incidence rate in this study was 0.0277 cases per person-year.
Bernard and colleagues [15] focused on executive functioning 4 and 6 months after ACS. At baseline, 36.1% of patients presented impaired executive function. At follow-up, 76.9% of the impaired improved their performance and 34.8% of the unimpaired became impaired. In consequence, at 6 months, 24.2% were classified as “impaired”, 30.3% as “transient impaired” and 45.5% as “cognitively normal” [15]. We have found an incidence rate of 0.416 cases per person-month in this study.
Regarding the neurocognitive functions most affected in ACS, this systematic review points to alterations in verbal memory, language and executive functioning.
In comparison to TIA and minor stroke, ACS patients did worth on MMSE recall on both 1 and 5 years and on repetition at 1 year. At 5 years, ACS patients had greater impairment on MoCA recall and language items than cerebrovascular groups [14]. In this study the number of vascular risk factors was associated to higher rates of cognitive impairment. Executive functioning it is also compromised. However, the determination of executive functioning was made only through the Trail Making Test B, thus limiting the wide use of this term on the context of ACS. Difficulties in working memory and secondarily in task-switching abilities seem to be more suitable to characterize these patients. These alterations are associated to an increased functional connectivity in medial-orbito-frontal region [15].
The variability of prevalence rates of neurocognitive dysfunction in ACS across studies may be due to the use of different neuropsychological instruments. None of the four selected studies used the same neuropsychological test. Testing was preformed through several instruments such as the St Louis University Mental Status [12], Short Portable Mental Status Questionnaire [13], MMSE and MoCA [14] and the Trail Making Test [15]. Variations between studies concerning the time of the neuropsychological assessment, demographical and clinical characteristics (e.g. treatment, type of ACS, comorbidities) of the samples, may also contribute to the observed differences. In fact, these variations between studies make the determination of a neurocognitive profile in the ACS very difficult.
In the future, further studies must clarify the neurocognitive tests that are more sensitive to ACS and its resilience and vulnerability factors [16]. The inclusion of more extensive neurocognitive measures into epidemiological studies, may offer the opportunity to track trajectories of domain-specific decline [16]. The study of the interaction between risk factors, biomarkers, behavioral and environmental aspects as well as the clinical features of ACS and its treatment on brain and neurocognitive functioning, should also be clarified [17]. Unveiling these interactions may point to new therapeutic targets aiming to prevent brain dysfunction and neurocognitive impairment [17].