Profile Characterization of Volatile Organic Compounds on in vitro Propagated Plants of Clinopodium Odorum and it’s Comparison with the Wild Plant
The aim of this work was to study the effect of the in vitro culture conditions of plants of Clinopodium odorum on the production of volatile organic compounds (VOCs), and to identify any differences with plants that grow wild. Plantlets were grown with different concentrations and combinations of growth regulators and different media conditions, with or without plant hormones. The VOC profiles of wild and in vitro cultivated plants were determined by HS-SPME/GC-MS, and the results were interpreted by performing statistical tests (principal component analysis (PCA) and conglomerate analysis (CA)). There was a wide variability in the VOC compositions depending on the growing conditions, with (-)-Menthone and Pulegone being the predominant VOCs produced in the most of the media studied. However, Pulegone showed a higher sensitivity to the nutrient composition media, with biosynthesis being favored by low salt concentrations. Moreover, pulegone was accumulated at higher concentrations on Murashige and Skoog (MS) and Gamborg (B5) media at half-strength salt media (MS1⁄2 and B51⁄2), which was dependent on the PGRS and nutrient addition. In conclusion, our present study shows that the micropropagation of C. odorum through in vitro axillary shoot proliferation is a reliable method for the rapid multiplication of this species, which allows the production of VOCs such as those found in spontaneous plants. In this way, we were able to select the most suitable chemotype.
Keywords: Tissue culture; Volatile organic compounds; HS-SPME/GC-MS; Menthone; Pulegone
List of abbreviations: Tissue culture; Volatile organic compounds; HS-SPME/GC-MS; Menthone; Pulegone
List of abbreviations: VOCs: Volatile Organic Compounds; HS-SPME: Head Space-Solid Phase Micro Extraction; GC-MS: Gas Chromatography Coupled Mass Spectrometry; KI: Kovat´S Retention Indices; PCA: Principal Component Analysis; CA: Conglomerated Analysis; ANOVA: Analysis of Variance; MS: Murashige-Skoog medium; B5: Gamborg medium; SH: Schenk and Hildebrandt medium; WP: Lloyd and Mc Cown medium; PGRS: Plant Growth Regulators; BA: Benzyladenine; GA3: Gibberellic Acid; IBA: Indole-3-Butyric Acid; BAP: Benzylaminopurine; NAA: 1-Naphthaleneacetic Acid; IAA: Indoleacetic Acid
Clinopodium odorum (Griseb.) Harley (Lamiaceae) [synonymy: Satureja odora (Griseb.) Epling] is a shrub of up to 2 meters tall and is native to Bolivia and Argentina. It has a pleasant aroma and grows wild in the mountain range of central Argentina. This shrub is also known as “muña-muña”, “salviolora” or “peperina”, and is found in undisturbed sites of mountain slopes, at between 1500 and 2000 m.a.s.l [1].
The fresh herb is used as a flavouring agent in food, and has gained considerable importance in traditional medicine of the local inhabitants for being anticatarrhal, antispasmodic, stringent, carminative, diuretic, stomachic, anti-oxidant, antiacidic, and soporific, and useful as a stimulant, vermifuge, menstrual suppressor and laxative, or in the treatment of flatulence, colic, altitude sicknesses, headache and stomach-ache, as well being anti-spasmodic and an aid in parturition [2,3].
It’s production depends entirely on the collection of wild plants, with this species being subject to a high extraction pressure resulting from its use as an herbal component of yerba mate composite (a traditional Argentine infusion), folk medicine and in the preparation of “Amargos serranos” beverages [4-6]. Because its collection and trade represent a source of income for local populations, enormous efforts need to be made to prevent its extinction [7]. With this goal in mind, we have recently developed a technique for the in vitro propagation of C. odorum [8].
Plants derived volatile organic compounds (VOCs) are one of the best studied groups of secondary metabolites, with over 1000 volatiles having been identified [9]. VOCs fulfill important functions, such as acting as signaling molecules, phytoalexins, and attracing pollinators [10]. However, due the high toxicity of these compounds, they are biosynthesized and stored at specific locations of the plant in specialized structures [11-13]. Thus, the occurrence of these structures is a key factor in terpene biosynthesis. The glandular trichomes, the secretory cavities and the idioblasts are found among the numerous types of specialized secretory structures responsible for the production and storage of these secondary metabolites [14]. According to Gottlieb and Salatino, the VOC production and the secretory structure formation are closely connected, with these compounds being related to plant defense and pollinator attraction among other ecological functions [15]. As secondary metabolite groups, these substances play an important role in the plant’s fitness under environmental changes. For this reason, a common problem that occurs in aromatic plant cultivation is the quantitative and qualitative variations in the production of VOCs in response to environmental factors [16].
A more promising approach is to improve the productivity and quality of the volatile components by biotechnological production processes. This provides an alternative method for obtaining a large number of plants that offer possibilities for reducing the risk of extinction [17,17]. In addition, seed is the only means of propagating C. odorum, but seeds have a reduced probability of germination when adult plants are already growing in the area, thus reducing species dissemination [19].
Compared to field cultivation, in vitro techniques offer the possibility of a continuous production of large amounts of chemically uniform biomass, independent of wild resources and environmental factors [20]. As far as VOCs are concerned, in vitro cultivation also permits the possibility of obtaining novel compounds, which have not been previously reported in wild plants [20,22].
Since the accumulation of volatile constituents was shown to be positively correlated with cell differentiation, most experiments aimed at establishing in vitro plant systems for VOC production were carried out using organ cultures [24]. According to Prins et al., growth regulators, or plant hormones, stimulate plant growth and terpene biosynthesis in a broad number of aromatic plant species, which results in beneficial changes in the quality and quantity of the VOCs produced [25].
As growth regulators can influence the formation and development of VOC biosynthesis and storage structures, the effect of plant hormone application on secretor structure formation was observed during a study of cytokine effects on VOC production of Thimus mastichina. Fraternale et al. verified a higher yield of VOCs in the medium culture with benzyladenine (BA) in leaves of T. mastichina plants treated with BA, with there being a larger density of glandular hair in the post-secretory stage [26].
The in vitro propagation of C. odorum is an alternative for obtaining different proportions of volatile components. For this reason, the aim of this work was to study in vitro plants of C. odorum grown under different media conditions and to determine the VOC profiles using a recently published HS-SPME/GC-MS technique [27]. These results were interpreted using principal component analysis (PCA) and conglomerate analysis (CA), which can identify any differences with the wild plant.
In all experiments, the chemicals used were of analytical grade (Sigma-Aldrich, St. Louis, MO, USA and E. Merck, Darmstadt, Germany). All cultures were maintained under the following conditions: 25 ± 2 °C, 45-55% relative humidity and a 16 h photoperiod under cool white fluorescent light (30 μE m-2S-1) followed by 8 h of darkness. Explants were observed daily for the first week for signs of contamination and thereafter weekly for signs of growth and development. Each treatment (performed in triplicate) consisted of 20 seeds, with germination being monitored weekly for up to 10 weeks.
For the HS-SPME analysis, specimens of C. odorum (Griseb.) in the process of flowering-fruiting were collected in February 2012 in the Sierras Grandes of Córdoba, Argentina (35°32´384 S; 64°33´252 O; altitude: 1663 meters above sea level). A whole wild plant was deposited in the Marcelino Sayago Herbarium (Register Number ACCOR 401), Faculty of Agricultural Sciences, Catholic University of Córdoba. Seeds of this plant were collected during the months of February and March when the fruits are ripe, and kept at room temperature until the start of the experiments.
For in vitro germination, seeds were washed overnight in running tap water and then soaked at 1 mg/L of Gibberellic acid (GA3) during 12 hs. In this condition the physiological dormancy was broken and germination rates increased (here by 62.2%), as observed in a previous investigation [8]. Seeds were surface sterilized with a solution of 70% (v/v) ethanol for 2 min and rinsed 3 times with sterile distilled water, followed by immersion for 15 min in a solution of sodium hypochlorite (NaOCl) 1.5% (v/v) for 15 min, before being rinsed 3 times with sterile distilled water. These surface sterilized seeds were explanted onto MS medium (germination culture medium, supplemented with 3% (w/v) sucrose, 0.7 % (w/v) agar and pH 5.8) using 20 seeds/ flask. Germination rates were measured 15 days after starting the culture.
To increase stock populations for multiplication rate assessment, clonal plants (plants originally derived from seed) were initially propagated on media for 4-5 months, following a previous report [8].
Multiplication experiments were carried out using nodal segments with axillary buds from in vitro germinated seedlings aged in half-strength MS major and minor salts medium with a solution of 1mg/L of indole-3-butyric acid (IBA) (initiation medium), as shown in Figure 1.
The node potential regeneration with axillary buds (0.5-1cm) obtained from plants grown in the above in vitro culture conditions was evaluated in terms of principal shoot length, node number, shoot formation and survival rate by culturing on media containing 3% (w/v) of sucrose. A previous study carried out by Diaz et al. determined the best media for the optimal growth of in vitro plants of C. odorum [8].
Murashige and Skoog (MS) MS0 and half-strength salt medium MS1/2 1:1.5 of both growth regulators; SH, Schenk and Hildebrandt SH0 and SH 0.5:0.5; Lloyd and Mc Cown (WP) WP0 and WP 0.5:0.5; Gamborg et al. B5, B5,0, and at half-strength salt medium; B5,1/2 and 0.5:0.5 at different mg/L concentrations and combinations benzylaminopurine (BAP) and 1-naphtaleneacetic acid (NAA) mg/L were used for plant regeneration [28-31]. The pH media was adjusted to 5.6 with NaOH or HCl, prior to gelling with 0.7% (w/v) agar-agar, dispensing (10 ml) into culture tubes and sterilized by autoclaving (121 °C for 15 min.).
Analyses of the VOCs were performed by HS-SPME/GC-MS, according to Vazquez et al., 2014 [27]. Samples (100.0 ± 0.1 mg) of aerial parts (previously chopped up with a clean cutter) were placed in glass vials of 20 cm3, which were then sealed with Viton septa and aluminium seals provided by Supelco (Sigma-Aldrich, Argentina). The vials containing these samples were immersed in a thermostatic water bath at 40 °C (PolyScience 8005, accuracy 0.2 °C), and after 10 min, the SPME device (equipped with a DVB-CAR-PDMS fiber provided by Supelco) was inserted into the sealed vial by manually penetrating the septum and exposing the fiber to the sample headspace for 10 min. After extraction, the needle on the SPME manual holder was set to its maximum length in the GC injector, and the fiber was directly exposed to the hot injector at 250 °C for 5 min in a split less mode.
The identification of volatile components was performed using a gas chromatograph HP 5890 Series II equipped with a manual injection port operating in a splitless mode and coupled to an HP 5970 Mass Detector. The column used was an HP-5 capillary column (30m x 0.25 mm ID x 0.25 m film), and the working conditions were: injector: 225 °C; interface: 230 °C; gas carrier: He 99.99%; head pressure: 5 psi; initial ramp: 40 °C to 90 °C (2 °C/min); middle ramp: 90 °C to 130 °C (10 °C/min); final ramp: 130 °C to 200 °C (5 °C/min). The mass spectrometer was operated at 70 eV, and the spectra were recorded in the range of m/z 50 - 550 amu in the acquisition mode “scan-full.”
The data processing system used was the HP-MS ChemStation including the Wiley 275 and NIST databases. The volatile components were identified by comparing their mass spectra with library data (match ≥ 90%) and by the determination of the respective Kovat´s retention indices (KI), with alkane standards provided by Sigma-Aldrich. The Retention indices were compared with those reported in the databases of the NIST/EPA/NIH Mass Spectral Library with Search Program. The percentage composition was established by normalizing the peak area of the chromatogram with respect to the total area. All determinations were performed in triplicate, and the variation coefficient was less than 5%.
All determinations were performed in triplicate, and significant differences were assessed by analysis of variance (ANOVA) and the Tukey HSD test (significance level of 5%). To identify any differences among the samples according to their chemical profiles (degree of clustering), a Principal Component Analysis (PCA) and cluster analysis (CA) were performed using the Infostat software (2014) version, Faculty of Agricultural Sciences, UNC).
Different nutrient media for growth culture conditions with or without hormones were tested for 8 weeks to determine a suitable starting C. odorum nutrient medium, which was supplemented with 3% (w/v) sucrose. WP0 free-hormone medium contains a proportion of inorganic nutrients that satisfies the nutritional factors as well as the physiological needs of in vitro culture plant species. However, the optimum growth depended on the addition of plant growth regulators (PGRS) [8].
The plantlets that grew on WP with the addition of BAP and NAA at 0.5:1 or 0.5:1.5 mg/L; MS0 and at half-strength salt medium: MS1/2 1:1.5; SH: SH0 and SH 0.5:0.5; WP: WP0 and WP 0.5:0.5; B5, 0 and at half-strength salt medium B51/2 at 0.5:0.5 promoted a higher plantlet elongations (Table 1). However, the optimum number of nodes was obtained in plantlets grown on MS1/2 with the addition of 1:1.5 of BAP and NAA (Table 2 and Figure 2) [8]. The WP and SH media have a lower salt concentration especially of NO3- and K+ , and for this reason it was not necessary to reduce the nutrient medium concentration. However, half-strength salt media of MS and B5 had to be used. The addition of PGRS produced different plant growth C. odorum responses, but using 0.5 mg/L of both plant growth regulators was found to be the best concentration.
The survival percentage (%) of C. odorum explants on different culture media combined with the addition of PGRS revealed the best optimum response on media with 0.5:0.5 of both growth regulators or on WP0 without PGRS. However for in vitro nodal cuttings, an efficient survival percentage was obtained for plantlets grown on free-hormone WP0 medium (Table 1).
The basic components of plant tissue culture media are the mineral nutrients, with the speed of tissue growth and the extent and quality of the morphogenetic responses being strongly influenced by the type and concentration of nutrients supplied [32]. The potential benefits of optimizing the nutrient component of culture media for a particular response are well documented for a wide range of species and applications. In order to determine optimal growth and morphogenesis processes it’s may vary between different plants according to their nutritional requirements [33].
The interaction of the mineral nutrients and plant growth regulators (PGRS) is a particularly intriguing relationship, which was evaluated to determine the effects of different PGRS. Consequently, it was necessary in some cases to reduce their concentrations or even eliminate them, as previously reported by Preece and Norton [34,35]. As there are 13 mineral elements essential for plant growth, the experimental determination of optimal nutrient levels is complex [36-38]. It is also known that the steps in plant tissue culture protocols can impose different type and composition of PGRS. In many plants, a balance between two groups of PGRS (auxins and cytokinins) is important, as these plant hormones regulate cell division and elongation and determinine morphogenesis [39,40].
An adequate assessment of the suitability requirements of nutrients and PGRS was carried out in a previous study [8]. This introduced the broad spectrum experiment, which determined that the best plant growth conditions were found on Murashige and Skoog (MS), MS0 and half-strength salt medium MS1/2 1:1.5 of both growth regulators; SH, Schenk and Hildebrandt SH0 and SH 0.5:0.5; Lloyd and McCown (WP) WP0 and WP 0.5:0.5; Gamborg et al. B5, B5,0, and for half-strength salt medium; B5,1/2 and 0.5:0.5 at different mg/L concentrations and combinations.
Figure 3 outlines the major components observed by HS-SPME/GC-MS in the wild and in vitro cultured plants of C. odorum. In addition, all the VOCs detected on each sample are shown in Table 3.
In the wild plant sample, the following volatile components were found (Figure 3a): pulegone (52 ± 3%), cis-isopulegone (12.1 ± 0.7%) and menthone (9.1 ± 0.4%). Furthermore, there were appreciable amounts of germacrene D (2.87 ± 0.06%), bicyclogermacrene (2.4 ± 0.1%), isomenthone (2.18 ± 0,B07%), δ-cadinene (1.43 ± 0.06%), linalool (1.40 ± 0.04%), piperitone oxide (1.32 ± 0.08%) and limonene (1.16 ± 0.07%), with the remaining observed components being observed at amounts ranging from 0.97 ± 0.04% (bicycloelemene) to 0.05 ± 0.01% (cis-limonene oxide).
For MS1/2 (Control) (Figure 3b): The plantlets of C. odorum that grew in this medium, showed as the major components: pulegone (57 ± 2%), cis-isopulegone (14.5 ± 0.3%) and menthone (14.0 ± 0.4%). Moreover, these plantlets synthesized sufficient pools of isomenthone (3.9 ± 0.1%) and linalool (2.22 ± 0.04%). The remaining components, β-caryophylene and α-elemene, were found at a range of 0.91 ± 0.04% and 0.04 ± 0.01%, respectively.
For MS1/2 plus PGRS (Figure 3c): The plantlets that grew in this medium had as major components pulegone (60 ± 1%), cis-isopulegone (16.4 ± 0.3%) and menthone (9.8 ± 0.3%). Additionally, there were also appreciable amounts of isomenthone (2.0 ± 0.4%), linalool (1.6 ± 0.6%), β-caryophylene (1.38 ± 0.07%) and one unknown compound with a peak at 9.12 min (1.5 ± 0.3%) was also found. Germacrene at 0.52 ± 0.01% and α-elemene and epi-bicyclosesqui-phellandrene at 0.01 ± 0.01% values were synthesized by these in vitro plants.
For WP0 (Control) (Figure 3d): When the volatile fractions of C. odorum plantlets were characterized, pulegone (56 ± 2%), cis-isopulegone (16.1 ± 0.7%) and menthone (13.18 ± 0.03% were found. Isomenthone (4.09 ± 0.07%), linalool (2.3 ± 0.1%), piperitone oxide (1.27 ± 0.06%) and limonene (1.0 ± 0.1%) were also produced. The rest of the components were found at a range of 0.56 ± 0.04% (β-caryophylene) and 0.01 ± 0.01% (carvacrol) (Table 1).
For WP plus PGRS (Figure 3e): The ability of the C. odorum plantlets to synthesize the following volatile compounds: pulegone (53 ± 1%), cis-isopulegone (17.3 ± 0.4%) and menthone (13 ± 1%) was observed in this medium. Isomenthone (4.36 ± 0.06%), linalool (1.92 ± 0.06%) and one unknown compound with a peak at 7.89 min (1.18 ± 0.06%) were also found. The rest of the components were produced at a range of 0.95 ± 0.01% (β-caryophylene) and 0.01 ± 0.01% (α-cadinene).
For B51/2,0 ( Figure 3f): The presence of pulegone (59 ± 1%), cis-isopulegone (18.6 ± 0.3%) and menthone (9.3 ± 0.1%) was observed in plantlets of C. odorum. Additionally, there were also appreciable amounts of isomenthone (2.1 ± 0.1%), linalool (1.83 ± 0.03%) and α-terpineol (1.13 ± 0.01%). β-caryophylene (0.86 ± 0.03%), aromadendrene (0.03 ± 0.01%) and α-gurjunene (0.03 ± 0.01%) were produced in plantlets that grew in this medium.
For B51/2 plus PGRS (Figure 3g): The presence of pulegone (64.0 ± 0.1%), menthone (12.8 ± 0.1%) and cis-isopulegone (10.2 ± 0.1%) was found in plantlets of C.odorum that grew in this medium. Isomenthone (3.5 ± 0.3%) and α-pínene (2.3 ± 0.1%) were also observed. The remaining components were found at a range of 0.70 ± 0.04% (β-myrcene y linalool) and 0.04 ± 0.01% (β-cubebene).
For SH0 (Control) medium (Figure 3h): Pulegone (59 ± 2%), cis-isopulegone (16.8 ± 0.3%) and menthone (13.18 were the major components founds in the plantlets of C.odorum. Both isomenthone (3.2 ± 0.1%) and linalool (1.41 ± 0.08%) were also synthesized in plantlets. The rest of the components were present at a range of 0.85 ± 0.01% (β-caryophylene) and 0.01 ± 0.01% (thymol).
For SH plus PGRS (Figure 3i): The major components of the plantlets C. odorum that grew in this medium were pulegone (57.7 ± 0.7%), cis-isopulegone (17 ± 1%) and menthone (10.5 ± 0.3%). Moreover, isomenthone (2.10 ± 0.08%), β-caryophylene (1.7 ± 0.4%), linalool (1.56 ± 0.07%) and α-terpineol (1.13 ± 0.03%) were synthesized on the in vitro plants of C.odorum. The remaining components were found at a range between 0.70 ± 0.01% (germacrene D) and 0.02 ± 0.01% (α-cadinol and epi-bicyclosesqui-phellandrene).
Biosynthesis of menthone occurred on SH0 (Control), MS plus PGRS, B51/2,0, WP plus PGRS, WP0 (Control), and MS1/2 (Control) media, while pulegone increased its synthesis for half strength concentration media.
The variability of the composition of volatiles in plants growing in different media can be explained by the fact that these secondary metabolites are produced as an induced response to various biotic and abiotic factors. Abiotic processes can be physical and chemical environmental factors such as drought, salinity, heat, cold, UV-B light, air pollution, heavy metals, nutritional deficiency and mechanical wounds, while biotic process are generally related to the defense mechanisms of the plant against the attack of microorganisms, insects or other plants [32,33].
Some reports emphasize that a variety of media and environmental factors can lead to qualitative and quantitative differences in the production of VOCs. Moreover, it has been found that stem cutting plants have many more VOCs than micropropagated plantlets. Considering that the biosynthesis of VOCs is generally more related with the primary photosynthetic process, the final composition of volatiles is also affected by the production method [42].
Our results revealed that the VOC content in the micropropagated and cutting wild plants had different percentages of the main components. In this study, the biosynthesis and emission of volatile compounds on in vitro C. odorum plants was a regulated process which was finely modulated by the medium nutrient composition and growth regulators. However, not all the cultured plantlets showed a positive correlation between the main volatiles (menthone and pulegone). (−) Menthone was the predominant monoterpene produced in the most of the media studied, while pulegone showed a higher nutrient concentration with a negative re ponse since it accumulated on MS and B5 for half-strength salt medium (MS1⁄2 and B51⁄2).
Close attention should be paid to the hormonal substances specially auxins and cytokins. Assessment of the hormonal (PGRS) requirements should consider the hormone types and their interactions. Taking into account that PGRS influence plant growth and development, thereby affecting the physiological and biochemical processes, or even the gene regulation, then there are a great number of ways in which applications of these compounds could alter the VOC production [43-45]. As the kinetin effects were stronger than those attributed to the effects related to growth and developmental changes, or to gland formation and density, an effect on the metabolism is suggested. Moreover, a study on the effects of different cytokinins in Cymbopogon species reported increases of 9 and 93% n the content of essential oil [46]. It was also observed that Lavandula spp produced a higher number of leaves in plants cultured in vitro for 8 weeks with 0.1 mg/L (IBA) [22]. In Melissa officinalis grown in a culture medium for 60 days with auxin and cytokinin complement (indoleacetic acid (IAA) 11.42 μmol L; BA 8.87 μmol / mg/L), an increase of 1.4 fold in nerol and 4.1 fold in geraniol was found. Plants grown on control medium and ex vitro, however, presented higher percentages of neral and geranial [42]. This summary of the results gives a broader spectrum of the volatile fraction components found on wild plants and those growing on plant tissue culture.
A comparative analysis was carried out with wild plants and C. odorum tissue culture. Considering that the composition of each volatile compound showed some similarities and differences, the results have a wide variability. For example, for the main component pulegone, the wild plant did not show any significant differences with plantlets grown on WP plus PGRS, MS1⁄2(Control) or SH plus PGRS. However, if the second main compound (menthone) is considered, the wild plant showed no significant differences with the plantlets grown on other media: B51/2 (Control), M1/2 plus PGRS or SH plus PGRS. Futhermore, the following components: Δ-3-carene, trans-β-ocimene, diosphenol, isobornyl acetate, α-methyl cinnamaldehyde, bicycloelemene, α-cubebene, α-ylangene, β-bourbonene, β-elemene, α-copaene, γ-elemene, aromadendrene, α-gurjunene, calarene, α-elemene, γ-muurolene, α-amorphene, germacrene D, γ-amorphene, bicyclogermacrene, γ-cadinene, β-cadinene, α-calacorene, spathulenol and δ–cadinol were synthesized in wild plants with marked significant differences when compared with those of the tissue culture.
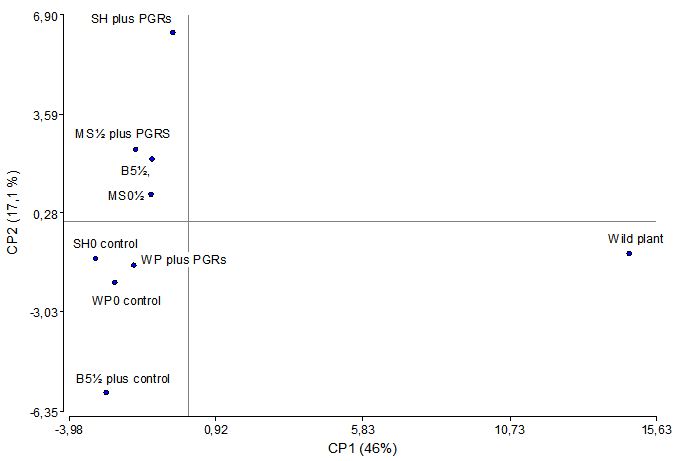
As the variability pattern found was repeated for each of the volatile compounds, it was necessary to perform a PCA and a hierarchical cluster (CA) analysis. The PCA allows a reduction in the number of variables by selecting the components that have the highest variability with a minimal loss of information. In this case, the first two principal volatiles explained 63% of the sample variance (data covariance structure), which reduces 64 variables to 2 main components. The two-dimensional space makes it easier to understand the results, where the importance of the variables may be inferred from the value of the coefficients and the distance from the origin (eigen values), which are shown in Table 2 for the principal components PC1 and PC2. As can be observed, there is a high prevalence of the variables Δ-3-carene, diosphenol, α-methylcinnamaldehyde, bicycloelemene. α-ylangene, β-bourbonene, β-elemene, α-copaene, β-cubebene, γ-elemene, aromadendrene, α-gurjunene, calarene, γ-elemene, δ-muurolene, α-amorphene, germacrene D, α-amorphene, bicyclogermacrene, β-cadinene, δ-cadinene, spathulenol and δ-cadinol along the first principal component. In contrast, along the second main component there is a great predominance of the variables α-terpineol, piperitenone, β-caryophylene, dehydroaromadendrane, β-selinene and cadine-1,4-diene. It is striking that the CP1 includes most of the variables in the wild plant that showed significant differences with all the tissue culture. Figure 4 displays the dispersion factor of the samples according to the first and two principal components (Table 4).
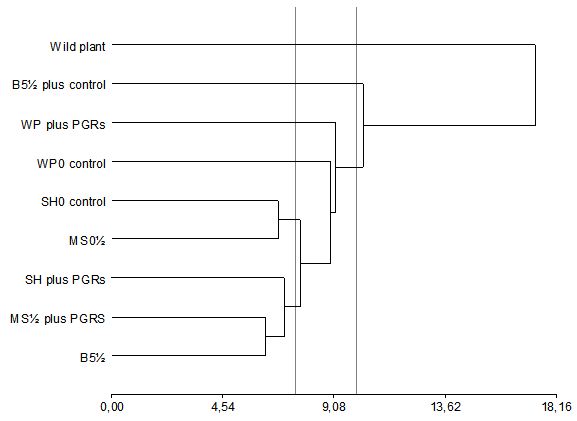
Hierarchical CA can identify classes within a data set were the intraclass variability is less than the interclass variability, with the starting point being a matrix distance between the objects that form a group. Figure 5 shows a dendrogram corresponding to the evolution of clusters based on the Euclidean distance of the samples according to their volatile composition (as a percentage). By setting an arbitrary cut-off criterion at a distance of 10 (58% of the maximum distance), the wild plant is separated from the rest. The outlying samples were plantlets cultured on WP plus PGRS and B51⁄2 plus PGRS, with the samples WP0, SH0 (Control), MS1⁄2(Control), SH plus PGRS, MS1⁄2PGRS and B51/2 forming a group that is isolated from the wild plant on one side and from the plantlet of C. odorum cultivated in B51⁄2 on the other.
For a cut off criteria set at 7.5 (43% of the maximum distance), plants grown in SH0 (Control) and MS1⁄2(Control) formed a group, while those grown in SH with PGRS, MS1⁄2PGRS and B51⁄2 formed another group, which is isolated from the other plants. The C. odorum tissue culture showed a positive correlation between the main volatile oil (menthone or pulegone) accumulation with macronutrient and micronutrient compositions. (-) Menthone was the predominant monoterpene produced in the most of the media studied (either media control or with the addition of growth regulators). Pulegone showed a higher sensitivity to the nutrient composition media, with its biosynthesis being favored for low salt concentrations. This component was accumulated at higher concentrations on MS and B5 for half-strength salt medium (MS1⁄2 and B51⁄2), independent of PGR addition to the culture media (nitrate, calcium, phosphate or sulfur, among others).
It is noteworthy that HS-SPME turned out to be a useful technique for the characterization of VOCs in in vitro micropropagated plants, considering that the amount of sample available was small. This represents a clear comparative advantage to the hydrodistillation methods for obtaining of the essential oils necessary to characterize the VOCs present in the samples. Moreover, the combination of the analytical data obtained by HS-SPME with the multivariate statistical analysis made it possible to identify any differences between the wild and micropropagated samples of C. odorum, enabling groupings to be established according to their chemometric profiles.
In conclusion, our present study shows that the micropropagation of C. odorum through in vitro axillary shoot proliferation is a reliable method for the rapid multiplication of this species, which permits the production of the VOCs found in spontaneous plants. In this way, we were able to select the most suitable chemotypes, which is a very important step for plant conservation in their natural habitats.


.JPG)
.JPG)
.JPG)
.JPG)
.JPG)