Properties of Tomato Peroxidase
Tomato peroxidase activity was investigated by using guaiacol and o-dianisidine as hydrogen donors. The effect of pH on the activity of enzyme was studied with 0.01 M potassium phosphate buffer (pH of 6.0, 6.5 and 7.0) and 0.01 M acetate buffer (pH of 4.5, 5.0 and 5.5). Peroxidase exhibited its maximum activity at pH 6.5 and pH 5.0 for guaiacol and o-dianisidine, respectively. At 0.1% (v/v) hydrogen peroxide, highest enzyme activity was found by guaiacol. Vmax and Km values for guaiacol were found as 0.52 abs/min and 0.66 mM, respectively. The Vmax and Km values for o- dianisidine were 1.14 abs/min and 0.16 mM, respectively Kinetics of peroxidase (POD) catalyzed reaction followed Michaelis-Menten model and low Km value was found by using o-dianisidine. Lower Km value of o-dianisidine indicates higher tendency of enzyme towards hydrogen peroxide.
Keywords: Tomato; Peroxidase; Kinetics; Activity
Peroxidases (donor: H2O2 oxido-reductase; EC 1.11.1.7) constitute a group of glycoproteins the main function of which is the oxidation of different substrates at the expense of H2O2. Peroxidases are ubiquitous, iron-containing enzymes, which oxidize phenolic compounds and related substances, using activated oxygen released from H2O2 or organic peroxides [1].
Peroxidase extensively distributed in higher plants (e.g., horseradish, turnip, fig sap), animals (e.g., tryptophan pyrrolase, iodine peroxidase of thyroid) and microorganisms (e.g., cytochrome c peroxidase of yeast [2]. Plant peroxidases can be subdivided into three subgroups (acidic, neutral and cationic) according to their isoelectrophoretic mobilities. Based on differences in primary structure, the plant peroxidase superfamily can be divided into three classes: I, II and III. Class I peroxidases include intracellular enzymes in plants, bacteria and yeast. Class II peroxidases are extracellular peroxidases of fungi. Class III comprises classical plant secreted peroxidases. Class III peroxidases enzymes have approximately 300 amino acids [3]. The peroxidases have been classified into two according to presence or absence of heme and non-heme peroxidases. With respect to PeroxiBase database, >80% of known peroxidase genes are reported to code for heme-containing peroxidases. Also, the nonheme peroxidases such as alkylhydroperoxidase, thiol peroxidase, NADH peroxidase constitute only a small proportion [4].
The enzyme is involved in many plant functions such as hormone regulation, defence mechanisms, indoleacetic acid degradation during maturation and senescence of fruits and vegetables and lignin biosynthesis. Because of its multiple functions, the enzyme is commonly found as several isoenzymes in plants. In the presence of peroxide, POD produces phytotoxic free radicals which react with a wide range of organic compounds (ascorbic acid, carotenoids and fatty acids), leading to losses in the colour, flavour and nutritional value of raw and processed foods [5].
There are four types of catalytic activity reported for peroxidases. These are the peroxidatic, oxidatic, catalytic and hydroxylation reactions. Peroxidase catalyzes oxidatic reactions (in which oxygen is the electron acceptor) as well as the classic peroxidatic reactions (in which hydrogen peroxide is the electron acceptor) [6,7]. The peroxidatic reaction, more generally thought to be of most physiological significance, has been studied more extensively than other three reactions.
Peroxidatic reactions occur in the presence of a wide variety of hydrogen donors, including p-cresol, guaiacol, resorcinol, benzidine and o-dianisidine. In the absence of hydrogen donor, peroxidase can convert hydrogen peroxide to water and oxygen although this reaction is some 1000 times slower than the peroxidatic and oxidatic reactions. Finally in the presence of certain hydrogen donors, such as dihydroxyfumaric acid, and molecular oxygen, peroxidase can catalyze hydroxylation of a variety of aromatic compounds notably tyrosine, phenylalanine, p-cresol and benzoic acid. The metabolism of phenolic substances is a particular importance to the quality of postharvest fruits and vegetables in that they may act as effectors of hormone metabolism, intermediates in lignin biosynthesis and may result in discoloration resulting from their enzymic or non-enzymic oxidation. The aim of this study was to investigate the kinetic properties of tomato peroxidase by using guaiacol and o-dianisidine as hydrogen donors.
Fresh ripened tomatoes (Solanum lycopersium, cultivar: SC 2121) were purchased from a local market. o-Dianisidine, guaiacol and hydrogen peroxide were obtained from Sigma–Aldrich Chemie GmbH, Germany. The stock solution of o-dianisidine was prepared daily in 100% methanol and solutions of other reagents, guaiacol and hydrogen peroxide were prepared using distilled water. The common reagents used were all reagent grade (Merck KGaA, Darmstadt, Germany).
Tomatoes were washed and cut into small pieces and blended in Waring blender at high speed for 30 sec with 20% (w/v) water addition at 4 ˚C . The slurry was filtered through two layers of cheese cloth and the filtrate was centrifuged for 10 min at 11000 rpm in an Eppendorf model 5810 R centrifuge at 15 ˚C [8]. The supernatant was kept frozen approximately at -20 ˚C and used as the enzyme source.
POD activity of tomato was measured using guaiacol and o-dianisidine as hydrogen donors. All analysis were done at least in dublicate. The changes in absorbance were read by using PerkinElmer Lambda 25 UV-VIS spectrophotometer at 25 ˚C. The wavelengths of 470 nm for guaiacol and 460 nm for o-dianisidine were used for activity measurements. Kinetic parameters such as Km and Vmax were determined using Lineweaver-Burk plot.
POD activity was determined spectrophotometrically as the change in absorbance at 470 nm. The reaction mixture contained 1.6 mL of 0.01 M KP buffer (pH 6.5) containing 1 mL 1.0% (v/v) guaiacol; 0.3 mL of 0.1% (v/v) H2O2 and 0.1 mL enzyme extract [9,10]. For the determination of kinetic parameters, H2O2 concentrations from 0.0987 mM to 9.78 mM were used at 75.4 mM guaiacol concentrations.
POD activity was determined spectrophotometrically at 460 nm as described by Robinson et al, (1989) [11]. The reaction mixture consisted of 2.7 mL of 0.01 M acetate buffer (pH 5.0); 0.1 mL of 0.1% (v/v) H2O2, 0.1 mL of 0.05% (w/v) o- dianisidine in methanol and 0.1 mL enzyme extract [12]. For the determination of kinetic parameters, H2O2 concentrations from 0.0653 mM to 1.956 mM were used at 6.8 x 10-5 mM o-dianisidine concentrations.
All experimental data were analyzed using the ANOVA and Duncan’s multiple range tests by the SPSS 9.0 (SPSS Inc., Chicago, IL) computer program. Unless otherwise noted in the text, a P<0.05 level was used where values were considered as being significantly different.
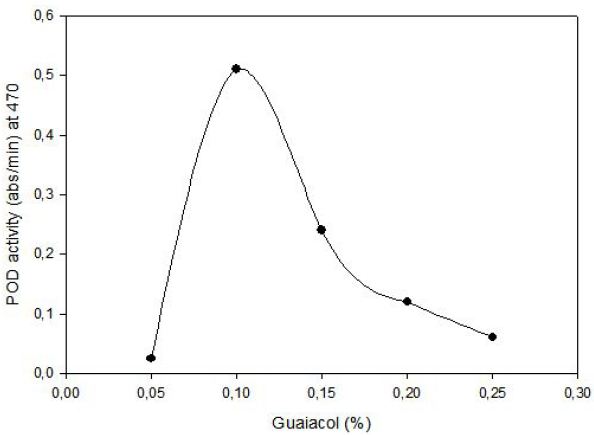
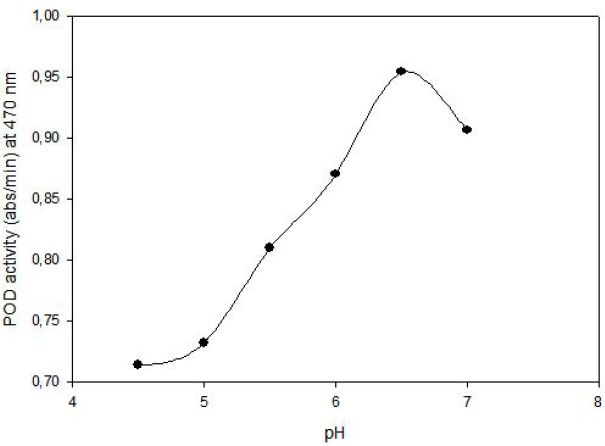
POD activity of tomato extract was determined by using guaiacol (2-methoxyphenol) as hydrogen donor. The increase in absorbance as a result of colored products of oxidized guaiacol was measured at 470 nm. Highest activity was observed at 0.1% (v/v) guaiacol and 0.1% (v/v) hydrogen peroxide (Figure 1). The effect of pH on the activity of enzyme was studied with 0.01 M potassium phosphate buffer (pH of 6.0, 6.5 and 7.0) and 0.01 M acetate buffer (pH of 4.5, 5.0 and 5.5). Peroxidase exhibited its maximum activity at pH 6.5 (Figure 2).
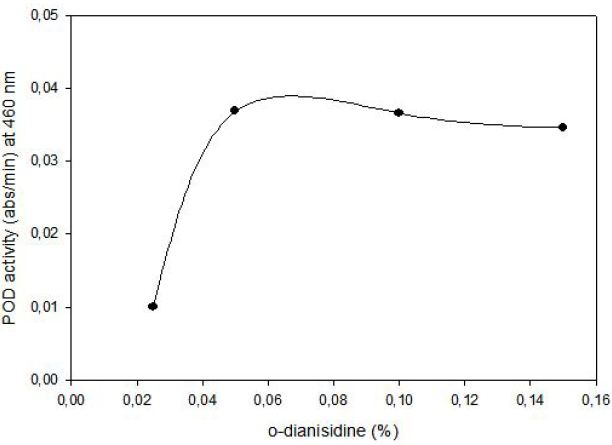
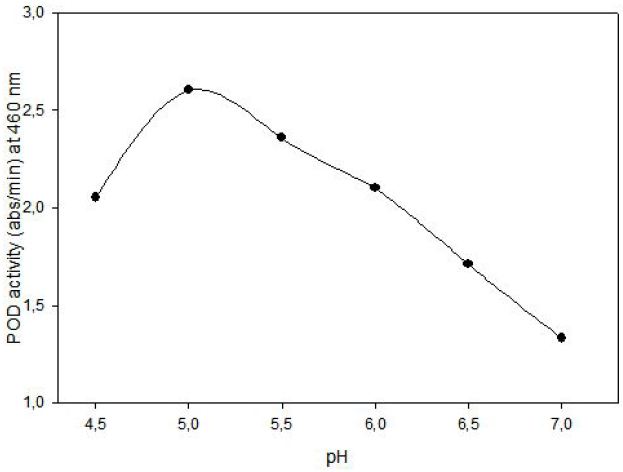
POD activity of tomato extract was determined by using o-dianisidine (3,3’-dimethoxybenzidine) as hydrogen donor. Hydrogen peroxide reacts with o-dianisidine in the presence of peroxidase to form a colored product. The increase in absorbance as a result of colored products of oxidized o- dianisidine was measured at 460 nm. Highest activity was observed at 0.05% (w/v) o-dianisidine and 0.1% (v/v) hydrogen peroxide (Figure 3). The effect of pH on the activity of enzyme was studied with 0.01 M potassium phosphate buffer (pH of 6.0, 6.5 and 7.0) and 0.01 M acetate buffer (pH of 4.5, 5.0 and 5.5) at fixed substrate (0.326 mM H2O2) and o-dianisidine (6.8x10-5 mM) concentrations. Maximum enzyme activity was observed at pH 5.0 (Figure 4).
Kinetics of tomato POD was determined by using guaiacol and o-dianisidine as hydrogen donors. By guaiacol, peroxidase activity was determined by KP buffer (pH 6.5) at 470 nm and by o-dianisidine it was determined by acetate buffer (pH 5) at 460 nm.
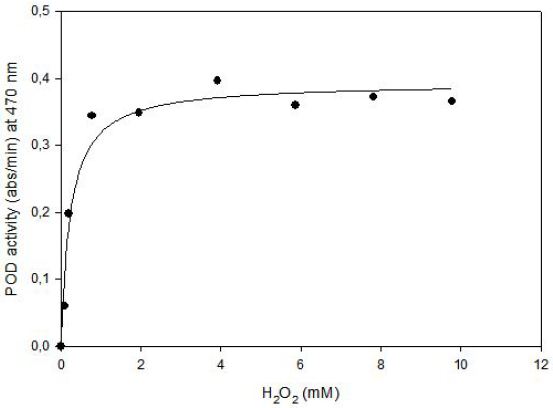
Kinetics of tomato POD was studied at 75.4 mM guaiacol and 0.0987 mM to 9.78 mM hydrogen peroxide concentration at fixed enyzme concentration. Data obtained from experiments was used for nonlinear equation of (y = ax / (b+x)) by using Sigma Plot 2000 ( Jandel Sientific, San Francisco, USA), where a = Vmax (abs/min) and b = Km (M). Then predicted data and equation’s constants were calculated. Experimental data and predicted data from the model were compared with correlation coefficients (r2), that was found as 0.97. (Figure 5) shows that enzyme follows Michaelis Menten Kinetics in the substrate concentration ranges of 0.0987 mM to 9.78 mM.
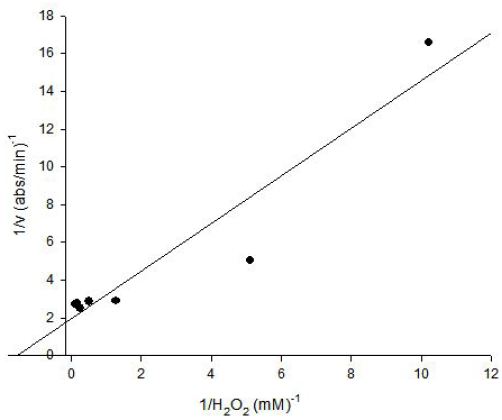
The Lineweaver-Burk plot was obtained as linear and linear correlation coefficient (r2) was found as 0.90 and Vmax and Km values were found as 0.52 abs/min and 0.66 mM, respectively (Figure 6) [13]. Km value for hydrogen peroxide by using guaiacol as hydrogen donor was lower than the value of 4 mM for tomato peroxide and 7.2 mM for green pea [14].
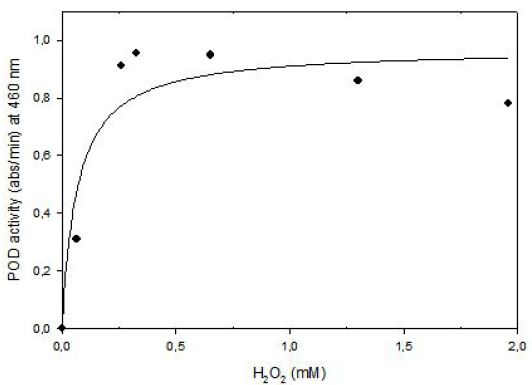
Kinetics of tomato POD was studied at 6.8 x 10-5 mM o-dianisidine in methanol and 0.0653 mM to 1.956 mM hydrogen peroxide concentration at fixed enzyme concentration. Data obtained from experiments was used for nonlinear equation of (y = ax/(b+x)) where a = Vmax (abs/min) and b = Km (M). Then predicted data and equation’s constants were calculated. Experimental data and predicted data from the model were compared with correlation coefficient (r2) that was found as 0.91(Figure 7). shows that enzyme follows Michaelis Menten Kinetics in the substrate concentration ranges of 0.0653 mM to 1.956 mM.
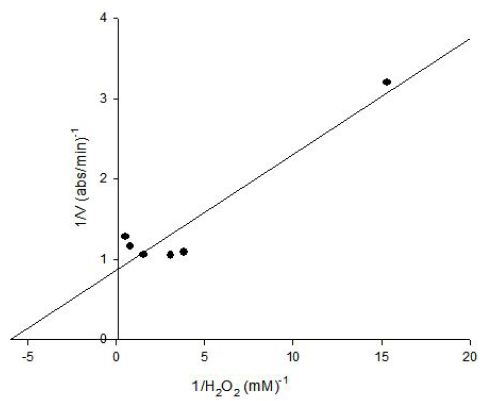
The Lineweaver-Burk plot was obtained as linear and linear correlation coefficient (r2) was found as 0.91. Vmax and Km values were found as 1.14 abs/min and 0.16 mM, respectively (Figure 8). Km value for hydrogen peroxide by using o-dianisidine as hydrogen donor was found as 7.7x10-3 mM for carrot peroxidase [15].
Enzyme activity is generally defined as the amount of enzyme that caused an absorbance change of 0.001 per min under experimental conditions [16]. Vmax is the maximum rate of reaction which occurs when the enzyme is completely saturated with substrate, Km is a Michaelis-Menten constant and is an essential parameter for characterization of a certain enzyme-substrate couple. A low value of Km indicates a high affinity to the enzyme for its substrate [17].
Vmax/Km ratio is called catalytic power and is a good parameter for finding the most effective substrate [18]. The Km values for guaiacol and o-dianisidine were obtained as 0.66 mM and 0.16 mM. The lowest Km and the highest Vmax/Km ratio were obtained with o-dianisidine. Lower Km value indicates higher tendency of enzyme towards hydrogen peroxide. As a result, when o-dianisidine was used as hydrogen donor, tomato POD showed higher tendency towards hydrogen peroxide. Also, when o-dianisidine was used as hydrogen donor reaction rate was higher than guaiacol. Because Vmax of o-dianisidine was 2.2 times higher than that of guaiacol with same enzyme concentration.