Proximate Composition and Microbial Quality of Clarias Gariepinus Subjected To Four Different Methods of Processing
Freshly harvested Clarias gariepinus were purchased from a reputable fish farm at Odogunyan in Ikorodu local Government area of Lagos State. Twenty five (25) fish samples of an average weight of 12.5±2.0kg were bought and divided into five (5) treatment levels at five (5) fish per treatment. The treatments were later subjected to different processing methods which include, smoking, Sundrying, salting and boiling and few specimens from each samples were analyzed for proximate composition and microbial count. Fresh C. gariepinus showed the highest total bacterial count of 1.4 x 10-3 cfu g-1 and smoked C. gariepinus showed the lowest total bacterial count of 1.6 x 10-1 cfu g-1. Bacterial pathogens were also isolated from all the processed fish samples and the isolates were characterized and identified as cocci and Baccilus.
The proximate analyses showed that boiled C. gariepinus had the highest fat content of (1.48%) and also had the lowest protein content (38.2%). Boiled C. gariepinus had the highest moisture content (62%) whereas fresh C. gariepinus had the highest crude fibre and lowest ash content (4.86 and 2.89%) respectively.
The boiled fish sample had less percentage of crude fibre which is responsible for its faster digestibility when compared with that of the salted C. gariepinus fresh sample.
Keywords: Clarias gariepinus; Crude Fibre; Protein; Fat; Ash
Fish serves as sources of protein, vitamins and minerals, and contain some essential nutrients required in small quantity for supplementing both infants and adults diet. In Nigeria, fish is served fresh or smoked in the nutrition and form as much cherished delicacy that cut across socio- economic, age, religious and educational barriers [1,2].
Fish is one of the important sources of protein to the large teeming population of Nigeria. Fish serves as one of the cheapest protein in human diets as it provides 55% of the dietary intake of animal protein to the average Nigerian. As reported by Adekoya and Miller. In rural areas fish and fish products constitute more than 60% of the total protein intake in adults [3,4].
Fish processing serves as one of the method of giving the fish product a form that is attractive to the consumers and also extend the storage life of the fish. The organoleptic, and flavor characteristics of processed fish to be stored should ensure full healthy safety of the product, proper sanitary conditions as well as rendering it impossible for the development of harmful micro-organisms and toxins [5].
Davis suggested appropriate processing technologies to enable this contribute to increase economic and the preservation practice adopted after capture affects the degree of spoilage of the fish [6,7].
Abraha, et al. suggested that Fresh fish after capture should be properly handled if the keeping quality and shelf-life are to be improved reasonably [8]
With these there will be a reduction in the period the fish will remain in rigor or stiffened, thereby, accelerate bacteria attack and spoilage.
One of the major reasons for fish drying is to reduce the moisture content of the fresh fish material to such a minimal level, that the quantity of moisture left will not support the growth of microbial organisms that feeds on the tissues; this also plays a major role in fish meal production. This level of drying is also considered appropriately low to stop the chemical degradation of the fish materials. Petterson and Ranjitha, also recommends that fish could be dried from 45% and up to 60% moisture content to a level of about 10% or less moisture content [9].
Fish drying process is one of the methods that mostly affect the protein quality which when exposed to high temperature for long period causes damages to the proteins that affects the nutritional quality of the fish. When the fish temperature is kept below 70 °C during this process of exposure, very little quality of the fish protein is affected. Little impact on the protein quality is felt when the fish are exposure to high temperature for a very short time of about (1 to 10mins). From research on salmon and milk fish, some special low temperature fish meals have been discovered to give a higher feed intake and better growth [9,10].
Nutrient content have been said to vary with fish species. Limited data have been discovered on the nutritional composition of fish species commonly consumed by the poor in developing countries of Asia and Sub-Saharan Africa.
According to Joseph, et al. who conducted a research on the effect of different preservation methods on the nutritional quality of Clarias gariepinus revealed that frying serves as the best means of preservation, but nutritionally reduced the moisture, ash and protein below the FAO acceptable limit. The fat and fibre content is increased above the acceptable limit [11].
Hence, the need for this study to evaluate the proximate composition and microbiological quality of Clarias gariepinus subjected to four different methods of processing.
The freshly harvested fish samples (clarias gariepinus) were bought from fish farm at Odogunyan in Ikorodu Local Government area of Lagos State. Twenty five (25) fish samples of an average weight of 12.5 ± 2kg was bought and divided into five treatment levels at five (5) fish per treatment level which include 4 methods of fish processing with treatment one (1) standing as the control.
The treatment levels were as follows T1 (fresh fish), T2 (Sundried), T3 (Smoked), T4 (Salted) and T5 (Boiled Fish).
Prior to these processing methods, the fish groups were salted using the procedure outlined below. The fish was clear with clean water and soaked for 1hr in not too strong brine; the brine was made by dividing 300g of salt in every four litres of water. By submerging the fish in this brine, the blood and slime are removed. The fish were then removed after some minutes and washed with clean water, after washing; the fish were placed in a saturated brine solution of 3.0-3.5kg of salt per 10L of water. Treatment 2 fish were placed on a clean band with clean washed stones on top of the container until the fish were covered by the brine. The fish were left for 6hrs in the brine. After the 6hrs, they were taken out of the brine and placed on a wire /net mesh rack to drain, taking care not to let the fish overlap while on the rack to facilitate faster moisture removal. The fish were placed on the drying rack and allowed to dry. The salting method described in the foregoing was carried out for the four processing method except that of the control and the fish in treatment four were later salted heavily to allow for total draining of water content. The fish were placed in the drying rack and left for 2 weeks till the end of the drying period. The fish were also smoked using the smoking kiln at a temperature range of 60-70 °C for 24hrs. At the end of the processing period, fish were stored away in clear polythene bags for proximate and microbiological analysis. 2gms of each treatment samples were measured out for use in the analysis.
These following media nutrient agar and nutrient broth (Hirmedia, India) were sterilized using an autoclave at 121 °C for about 15 mins and were employed in this experiment in accordance with the manufacturer’s manual specification.
One gram (1g) of each sample was dissolved in sterile de-ionized water and sequentially diluted. About 1 millilitre of each of the dilution were planted on plate count agar using the method of spread plate, and the medium was later incubated for 24 hours at 37 °C room temperature.. Each of the plate count agar was examined for the presence of colony and which were counted and recorded after incubation for 24hrs at 37 °C room temperature to obtain the total colony count in cfu g-1.
One gram (1gm) of each fish sample was sequentially diluted and 1ml of the right dilution was directly inoculated on a nutrient agar plates and the plates were later incubated at 30% for 24hrs. After the completion of the 24 hrs incubation system, a sterile wire loop was used in picking the isolate from the plate and was spread on a freshly prepared sterile nutrient agar plates, and this was later incubated at 30% for 24hrs to obtain clean pure cultures.
The clean pure cultures were refrigerated at 4 °C and the different isolates were characterised using the routine laboratory method of Cruikshawk, et al. [12].
The identification of the isolates was done using their following characteristics such as microscopic, cultural, physiological and biochemical.
Bergey’s Manual of Systemic Bacteriology was used in identification of the isolates subjected to standard culture, morphological and physiological techniques.
These following physiological test such as gram staining, catalase test, coagulate test, Methyl red test, voges-proskaeur test, indole test oxidase test, sugar fermentation test were used in identification of the isolates when subjected to and were later differentiated using the Bergey’s Manual of Systemic Bacteriology [13].
All samples of raw materials and finished products were stored for analysis in sealed containers. Prior to analysis, samples were grounded and allowed to pass through a 1mm screen. Moisture determinations were carried out on the material before and after grinding, as described below to prevent prolonged grinding.
Moisture is determined by the loss in weight that occurs when a sample is dried to a constant weight in an oven. About 2g of a feed sample was weighed into a silica dish previously dried and weighed. The sample was then dried in an oven at 105 °C for 24 hours, and allowed to get cooled in desiccators and weighed. The drying and weighing continues until a constant weight is achieved [14].
Since the water content of feed varied widely, ingredients and feed are usually compared for their nutrient content on moisture free or dry matter (DM) basis.
% DM = 100 - % Moisture.
Crude protein is determined by measuring the nitrogen content of the feed and multiplying it by a factor of 6.25. This factor is based on the fact that protein contains 16% nitrogen. Crude protein is determined by kjeldahl method. The method involves: Digestion, Distillation and Titration.
Digestion: 2g of the sample was weighed into a kjeldahl flask and 25mls of concentrated sulphuric acid, 0.5g of copper sulphate, 5g of sodium sulphate and a speck of selenium tablet were added. Heat was applied in a fume cupboard slowly at first to prevent undue frothing, and continue to digest for 45mins until the digester become clear pale green. This was left until it is completely cooled and 100ml of distilled water was added rapidly. The digestion flask was rinsed 2-3 times and the rinsing was added to the bulk.
Distillation:Markham distillation apparatus was used for distillation. The distillations apparatus were steamed up and about 10mls of the digest was added into the apparatus via a funnel and allowed to boil. 10ml of sodium hydroxide from the measuring cylinder was also added so that ammonia is not lost. This was later distilled into 50ml of 2% boric acid containing screened methyl red indicator.
Titration: the alkaline ammonium borate formed was titrated directly with 0.1 N HCL. The titre value which was the volume of acid used was recorded. The volume of acid used was fitted into the formula which becomes
VA = volume of acid used w = weight of sample
% crude protein = %N x 6.25.
The nitrogen value was therefore multiplied by 6.25 to get the weight of protein [14].
Ash is the inorganic residue obtained by burning off the organic matter of feedstuff at 400-600 °C in muffle furnace for 4hrs. 2g of the sample was weighed into a pre-heated crucible. And the crucible placed into a muffled furnace at 400-600 °C for 4hrs or until whitish grey ash was obtained. The crucible was then placed in the desiccators and weighed [14].
The organic residue left after sequential extraction of feed with ether can be used to determine the crude fibre, however if a fresh sample is to be used, the fat in it could be extracted by adding petroleum ether, stir, allowed to settle and decant. This was done three times. The fat free material was later transferred into a flask/beaker and 200ml of pre-heated 1.25% H2SO4 was added and the solution was gently boiled for about 30mins, maintaining constant volume of acid by the addition of hot water. The Buckner flask funnel fitted with whatman filter was pre–heated by pouring hot water into the funnel. The boiled acid sample mixture was then filtered hot through the funnel under sufficient suction. The residue was washed several times with boiling water (until the residue becomes neutral to litmus paper) and transferred back into the beaker. Then 200mls of pre–heated .25% Na2SO4 was added and boiled for another 30mins. This was filtered under suction and washed thoroughly with hot water and twice with ethanol. The residue was later dried at 65 °C for about 24hrs and weighed. The residue was then transferred into a crucible and placed in muffle furnace (400-600 °C) and ashed for 4hrs, then allowed to cool down in desiccators and weighed [14].
The organic residue left after sequential extraction of feed with ether can be used to determine the crude fibre, however if a fresh sample is to be used, the fat in it could be extracted by adding petroleum ether, stir, allowed to settle and decant. This was done three times. The fat free material was later transferred into a flask/beaker and 200ml of pre-heated 1.25% H2SO4 was added and the solution was gently boiled for about 30mins, maintaining constant volume of acid by the addition of hot water. The Buckner flask funnel fitted with whatman filter was pre–heated by pouring hot water into the funnel. The boiled acid sample mixture was then filtered hot through the funnel under sufficient suction. The residue was washed several times with boiling water (until the residue becomes neutral to litmus paper) and transferred back into the beaker. Then 200mls of pre–heated .25% Na2SO4 was added and boiled for another 30mins. This was filtered under suction and washed thoroughly with hot water and twice with ethanol. The residue was later dried at 65 °C for about 24hrs and weighed. The residue was then transferred into a crucible and placed in muffle furnace (400-600 °C) and ashed for 4hrs, then allowed to cool down in desiccators and weighed [14].
NFE is determined by mathematical calculation. It is obtained by subtracting the sum of percentages of all the nutrients already determined from 100.
% NFE = 100 – (% moisture + % CF + %CP + % EE + % Ash)
NFE represents soluble carbohydrate and other digestible and easily utilizable non-nitrogenous substances in feed [14].
The ether extract of a feed represent the fat and oil in the feed. Soxhlet apparatus is the equipment used for the determination of ether extract. It consist of 3 major components
1. An extractor: comprising the thimble which holds the sample 2. Condenser: for cooling and condensing the ether vapour 3. 250ml flask.
Procedure: about 150ml of an anhydrous diethyl ether (petroleum ether) of boiling point of 40-60 °C was placed in the flask, and 2–5g of the sample was weighed into a thimble and the thimble was plugged with cotton wool. The thimble with the contents was placed in the extractor, and the ether in the flask is then heated. As the ether vapour reaches the condenser through the side arm of the extractor, it condenses to liquid form and drop back into the sample in the thimble, the ether soluble substances are dissolved and are carried for at least 4hrs. The thimble was removed and most of the solvent were distilled from the flask into the extractor. The flask was then disconnected and placed in an oven at 65 °C for 4hrs, and allowed to cool in desiccators and weighed [14].
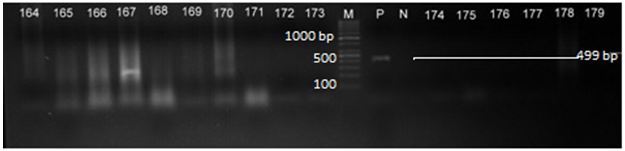
Total of 2 organisms were isolated from all the processed fish samples including the fresh sample of Clarias gariepinus. The 2 organisms were subjected to microscopial tests. And it was discovered that the 2 organisms were positive to Gram reaction, except in that of boiled fish sample that was negative in terms of bacilli organism. The isolates were identified to be cocci and bacilli. The values for total colony count for bacterial isolates is shown in Table; fresh Clarias gariepinus showed the highest total cocci bacteria count 1.4 x 10-3 cfu g-1 smoked Clarias gariepinus, had the highest Baccilli bacteria count while that of smoked C. gariepinus, sundried C.gariepinus, salting C.gariepinus and Boiling had the lowest cocci and bacilli bacteria count respectively.
The distribution of the bacteria species present in all samples is shown in Table 2 cocci and bacilli were present in fresh C. gariepinus, smoked C.gariepinus, Sundried C.gariepinus, Salted C.gariepinus while only cocci was present in Salted C. gariepinus.
The proximate analysis was done for the fish samples which included fresh, smoked, sundried, salted and boiled C. gariepinus. The percentages of the crude protein for the five samples were 67.6, 70, 70, 70 and 38.2 respectively. The percentage crude fibres were as follows 4.86, 0.65, 0.15, 4.72 & 0.28 respectively. The percentages of fat for the four samples were 0.60, 0.53, 0.66, 0.73 and 11.48% respectively. The percentage of ash included were as follows 2.89, 23.06, 25.35, 5.52 & 5.26 and the percentage of moisture were 61, 6.4, 9.5, 5.5 and 62 respectively (Table 3).
All the isolates of the present study were found to be positive (present) in all the treatments samples except in Boiled Clarias gariepinus (BCG) where the bacilli were absent. The isolates bacteria include species of the govern cocci and bacillus spp, this supports the previous work of ICMSF who reported that bacteria present in Fishes are normally amounted with those found in their natural environment and influenced by the season and the harvesting conditions. The proportion of the initial population can easily be changed after harvesting but this depends on the ability of those bacteria to adapt to the new condition [15].
Comparison of the total bacterial count of the Fresh, Smoked, Sundried, Salted and Boiled samples showed that fresh C. gariepinus had the highest colony count of 1.4 x 10-3, while that of smoked, dried, salted and boiled had the lowest total colony count of 1.6 x 10-1, 1.7 x 10-1, 1.8 x 10-1 and 1.9 x 10-1, which indicates that sundried, smoked, salted and boiled reduced the microbial load of the samples.
Sundried, smoked and salted had the same higher level of protein (70%) than the boiled and fresh fish (38.2 & 67.6%). The result shows that there was no significant difference (P > 0.05) in the value of the crude protein of the smoked, sundried and salted fish compared to that of the fresh fish, which serves as the control.
Increase in the level of protein of the smoked, sundried and salted fish may be as a result of the dehydration of the water molecules present between the proteins thereby, causing aggregation of protein and this resulting in the increase in protein content of the fish samples. Ogbonnaya and Shuba reported that protein nitrogen was not active during drying, so that protein content increased with the reduced moisture content in the fish samples [16,17].
Ash content in sundried fish (25.35%) was higher followed by that of the smoked fish (23.06%) and was lesser in other fish samples stated as follows. Fresh fish (2.89%), salted fish (5.52%) and boiled fish (5.28%) respectively. Clucas and Ward reported that the inorganic content remain as ash after the organic matter is removed by incineration. The result showed that there were no significant difference (P >0.05) in the level of ash of these two samples of fish salted and boiled when compared to that of the fresh samples [18].
The increase in the ash content observed in sundried and smoked fish may be as a result of the processing method employed.
Boiled fish samples had the highest fat content of 1.48% which may be as a result coagulation of fats after boiling and cooling. The amount of fat content observed in salted fish (0.73%) may be as a result of an increase in fat content during drying and this variation could be the result of evaporation of moisture content which is in agreement with the previous work of Ogbonnaya and Shaba [17].
Moisture content of the fresh and boiled fish samples were higher than that of the other fish samples (Sundried, Smoked, and Salted) as a result of dehydration of water molecule present in the 3 samples of fish.
Both the fresh and the smoked fish are hereby recommended for consumption because of its lesser percentage of crude fibre which is responsible for its faster digestibility compared to that of other source of protein.
This work supports the work of Eyo who reported that fish has a great digestibility [19].
Sundried, smoked and salted fishes also contain higher percentage of fat which is responsible for the supplies of omega–3 pufas for lowering blood cholesterol level and high blood pressure.
In general, there were great influence of Sundrying, smoking and salting on the proximate composition of Clarias gariepinus. These result showed that different nutritional components of fish undergo different changes at elevated temperatures.