Recovery of Non-Sugar Compounds from Bagasse Hydrolysates
Non-sugar compounds (e.g., organic acids, phenolic compounds, furfural, 5-HMF) released during the pretreatment of lignocellulosic biomass can have a negative impact on downstream enzymatic and microbial processes. However, if extracted and recovered, these compounds can serve as building blocks to numerous value-added chemicals, leaving behind a solution of fermentable sugars as potential carbon source. Six hydrophobic imidazolium-based ionic liquids ([OMIM][PF6 ], [BMIM][PF6 ], [OMIM][BF4], [HMIM][BF4], [OMIM][NF2] and [BMIM][NF2]) were evaluated in the liquid-liquid extraction and recovery of these non-sugar compounds from enzymatically hydrolyzed dilute ammonia pretreated energy cane bagasse hydrolysates. Energy cane bagasse was pretreated at 208 °C, for 36 min, at an ammonium hydroxide (28% v/v solution) to biomass to water ratio of 0.4:1:20, followed by enzymatic hydrolysis at optimum conditions for cellulase, xylanase, laccase, and Tween® 80.Phenolic compounds were removed from the hydrolysates by all six ionic liquids, followed by furfural and 5-HMF, and with formic acid and acetic acid failing to partition. Overall, [OMIM][NF2], [BMIM][NF2], [HMIM][BF4] and [OMIM][BF4] were most effective in extracting phenolic compounds, furfural and 5-HMF with minimal sugar losses. No more than two regenerations of [OMIM][NF2], [BMIM][NF2], [HMIM][BF4] and [OMIM][BF4] are recommended. The type of anion and cation as well as the attached alkyl chain in ionic liquids played critical roles in the final extractability of non-sugar compounds from biomass hydrolysates.
Keywords: Energy Cane; Bagasse; Ionic Liquids; Hydrolysate; Pretreatment
Lignocellulosic biomass, mainly composed of cellulose, hemicellulose and lignin, is an abundant and renewable resource that can be used in the production of bio-based fuels and chemicals [1].The recalcitrant nature of cellulose, which is guarded by hemicellulose and lignin, makes enzymatic conversion a challenge [2]. This puts emphasis on the value of developing an effective pretreatment to remove the lignin and solubilize the hemicellulose while preserving the maximum amount of sugars [3-7]. Among all types of pretreatments, ammonia-based pretreatments allow for the removal of lignin and for the preservation of the polymeric sugars [2,8]. However, harsh pretreatment conditions can promote the degradation of the lignocellulosic components. These results in the generation of non-sugar compounds (e.g., organic acids, phenolic compounds, furaldehydes) and their presence in the hydrolysates can have inhibitory effects on downstream processes, such as enzymatic hydrolysis and microbial fermentations [9]. Under severe pretreatment conditions, degradation of pentose and hexose sugars results in the formation of 2-furaldehyde (furfural) and 5-hydroxymethyl-2-furaldehyde (5-HMF), respectively [10]. Furfural can further degrade into formic and levulinic acids, and 5-HMF into formic acid [9]. Acetic acid can be generated from the hydrolysis of acetyl groups found in the hemicellulose. Phenolic compounds (e.g., gallic, 4-hydroxybenzoic, chlorogenic, vanillic, caffeic, syringic, coumaric, sinapic, trans-cinnamic) can be released from the partial degradation of lignin [11]. Under oxidative conditions, phenolic compounds can further oxidize into organic acids [11]. In addition to pretreatment, enzymatic hydrolysis also contributes to the formation and release of trapped lignocellulosic oligosaccharides, aliphatic and organic acids as well as furan derivatives and phenolic compounds [12-14].
The use of solvents or chemicals is the preferred option for the removal of the above-mentioned non-sugar compounds from various environments [15]. Some of these methods include chemical neutralization, over liming and the use of ionic liquids, polymers, flocculants, ion exchange resins, or activated charcoal [16-21].Once recovered, these non-sugar compounds can be used as platform chemicals in the production of biopolymers, biochemicals and pharmaceuticals, a sustainable alternative from petroleum-derived chemicals [22-25].
Imidazolium-based ionic liquids are a group of ionic liquids characterized by the presence of three different anions and two cations with different alkyl chains available for each of the anions. The presence of cations, anions and alkyl chains allow for their change in hydrophobicity. These salts can stay in the liquid form at ambient temperatures, have low vapor pressure and can solubilize most organic and inorganic compounds, thus making them great candidates for liquid-liquid extractions and substitutes to conventional extractions by conventional solvents [26]. The use of ionic liquids also addresses the various environmental concerns often associated with using conventional solvents [27]. One of the major concerns of working with ionic liquids is their production cost as compared to conventional solvents; however, ionic liquid recyclability can compensate for this cost-related challenge. The ideal ionic liquid should have a high selectivity and partition coefficient for all the main non-sugar compounds found in the hydrolysates. Ionic liquids should not remove or hydrolyze any sugars from the aqueous phase as sugar losses should be minimized. Furthermore, regeneration of the ionic liquid itself as well as the recovery of the extracted non-sugar compounds should be feasible. Several methods have been developed for the recovery and recycling of ionic liquids from various solutions and those include extraction, distillation, adsorption, membrane separation, and crystallization to mention just a few [28]. Among these methods, extraction and distillation are most commonly applied [28].
This study investigated the effect of six imidazolium-based ionic liquids (1-methyl-3-octylimidazolium hexafluorophosphate [OMIM][PF6 ], 1-butyl-3-methylimidazolium hexafluorophosphate [BMIM][PF6 ], 1-methyl-3 octylimidazolium tetrafluoroborate [OMIM][BF4], 1-hexyl-3-methylimidazolium tetrafluoroborate [HMIM][BF4], 1-methyl-3-octylimidazolium bis (trifluoromethyl sulfonyl) imide [OMIM][NF2], and 1-butyl-3-methylimidazolium bis (trifluoromethyl sulfonyl) imide [BMIM] [NF2]) on extracting of non-sugar compounds from dilute ammonia pretreated energy cane bagasse hydrolysates. Regeneration studies of the imidazolium-based ionic liquids were also evaluated.
Energy cane is a cross breed between commercial and wild sugarcanes with higher fiber content, lower water input requirement, higher cold tolerance, and higher biomass yield than sugarcane. Energy cane non-commercial variety Ho 02-113 was bred in Houma, LA through the collaboration between the United States Department of Agriculture-Agricultural Research Service (USDA-ARS) in Houma, LA and the Louisiana State University Agricultural Center Sugar Research Station in St. Gabriel, LA. Harvested energy cane was passed through a roller press (Farrel Corporation, Ansonia, CT) to remove the juice. The remaining solid material with moisture content close to 50% is referred to as bagasse. Bagasse was dried in an oven at 45 °C to a final moisture content of almost 10%. Partially dried bagasse was milled (Wiley Mill, Arthur Thomas Co, PA) and sieved (2 mm mesh sieve) prior to storage at -20 °C until further use. Milling of bagasse was performed to homogenize the bulk biomass and facilitate downstream feedstock handling, storage and treatment efficacy. All processes, regardless of feedstock type, require some form of comminution to convert the raw biomass into a feedstock suitable for industrial use [29]. Furthermore, in order to maximize the usable net energy of the resulting process, a primary goal of any comminution system must be to minimize the energy that is expended in the process.
Energy cane bagasse was pretreated in a 4L stirrer reactor (Autoclave Engineers, Erie, PA) at 208 °C, for 36 min, and at an ammonium hydroxide (28% v/v solution, Fisher Scientific, Pittsburgh, PA) to biomass to water ratio of 0.4:1:20. The pretreatment conditions used in this study had been previously optimized for maximum sugar yields using Response Surface Methodology (RSM) and the software Design-Expert 9.0.3 (State Ease Inc., Minneapolis, MN) [8]. The pretreated slurry was pressed to separate the liquid and solid fractions. The solid fraction was oven dried at 45 °C to reduce the moisture content below 10%. Composition analysis of energy cane bagasse was performed on untreated and pretreated biomass following NREL’s Laboratory Analytical Procedures (LAP TP-510-42618, 42619, 42622). NREL reference material 8491 (for sugarcane bagasse) was analyzed as an internal sample to ensure the accuracy of the procedures.
Cellulase (Cellic® CTec2) and xylanase (Cellic® HTec2) with the following optimum temperature (45–50 °C) and pH (pH 5.0–5.5) conditions were provided by Novozymes (Novozymes A/S, Bagasvaerd, Denmark). Cellulase activity of CTec2 (132 FPU/ml) and HTec2 (90.75 FPU/ml), and β-glucosidase activity for CTec2 (3229 IU/ml) and HTec2 (12.61 IU/ml) were measured according to the Ghose method [30]. Xylanase activity of Ctec2 (12100 IU/ml) and Htec2 (56000 IU/ml) were determined following the Bailey method [31]. Tween® 80 was added to improve cellulose digestibility and enzyme stability. Laccase was added to enhance enzymatic hydrolysis. Laccase from Rhus vernicifera was purchased from Sigma (Sigma–Aldrich, Inc., St. Luis, MO, USA). Laccase activity (50 IU/ml) was measured using syringaldazine as substrate as described by Ride [32]. Enzymatic hydrolysis of dilute ammonia pretreated energy cane bagasse was performed in Erlenmeyer flasks at 8% w/w solid loading (dry basis) in a 0.1 M sodium citrate buffer solution. The final pH was adjusted to 4.8 with concentrated hydrochloric acid. Optimum enzymes and surfactant concentrations were 19.39% (w/w glucan) CTec2, 12.04% (w/w glucan) HTec2, 46.32 IU laccase/g dry biomass, and 10.15% (w/w dry biomass) Tween® 80. Concentrations of CTec2, HTec2, laccase, and Tween® 80 were optimized for maximum sugar yields using RSM and the software Design-Expert 9.0.3 [33]. Erlenmeyer flasks were placed in a shaker incubator (Amerex Instruments Inc., Lafayette, CA) for 72 h at 50 °C and at 150 rpm. Samples were collected at 0 h (before the addition of enzymes and surfactant), 24 h, 48 h, and 72 h and kept at -20 ºC until further analysis. All collected samples were centrifuged at 10,000 rpm (Spectrafuge 24D, Labnet International Inc., Woodbridge, NJ) for 5 min and filtered (0.2 μm Syringe Filters, Environmental Express Inc., Mt. Pleasant, SC). Samples were diluted accordingly and analyzed for sugars, organic acids, 5-HMF, and furfural by high performance liquid chromatography (HPLC) as described below and for total phenolic compounds as described by Alvira, et al. Values are reported as the mean of three replicate with standard deviations [34].
Ionic liquids were purchased from Iolitec (Tuscaloosa, AL) and included 1-methyl-3-octy limidazolium hexa fluorophosphate ([OMIM][PF6 ]),1-butyl-3-methylimidazoliumhexafluorophosphate([BMIM][PF6 ]),1-methyl-3-octylimidazolium tetrafluoroborate([OMIM][BF4]), 1-hexyl-3-methylimidazolium tetrafluoroborate ([HMIM][BF4]), 1-methyl-3-octylimidazolium bis (trifluoromethylsulfonyl) imide ([OMIM][NF2]), and 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide ([BMIM][NF2]). The chemical structures of all six ionic liquids are shown in Figure 1. Ionic liquid to hydrolysate mass ratios of 1:3, 1:2 and 1:1 were evaluated. The ionic liquid to hydrolysate mass ratio of 1:1was chosen for further experiments as the other two ratios showed poor extraction results (data not shown). Samples from dilute ammonia pretreated energy cane bagasse hydrolysates were centrifuged at 10,000 rpm and filtered (0.2 μm) followed by the separate addition of equal volumes of each ionic liquid. Samples were stirred vigorously for 5, 10, 15, and 20 min and centrifuged for 5 min at 10,000 rpm. Two layers were formed with the hydrolysate remaining on the top layer and the ionic liquid at the bottom layer. Collected samples were analyzed for sugars, organic acids, 5-HMF, and furfural by HPLC as described below and for total phenolic compounds as described by Alvira, et al. Because greater sugar losses were observed after 10 min of stirring, liquid-liquid extractions of phenolic compounds, furfural, 5-HMF, and organic acids were carried out after 5 min of stirring [34]. The bottom layer (ionic liquid) was used in the regeneration studies. All the values are the mean of three replicates with standard deviations.
Regeneration of ionic liquids was performed to assess their reusability. Based on the method published by Fan, et al. ionic liquids were mixed with 0.1 M NaOH solution (stripping solution) for 30 min [35]. This step was repeated three times. Recovered ionic liquids were washed with distilled water to be neutral and remove any impurities. Washed ionic liquids were oven-dried at 75 °C for two days and re-used. All presented values are the mean of three replicates with standard deviations.
Collected samples were analyzed for sugars (glucose, xylose, arabinose, mannose) by HPLC (Agilent 1200 Series) with a BioRad Aminex HPX-P87P (PI), lead form, 300 mm × 7.8 mm (ID), 9 μm column at 80 °C and a differential Refractive Index Detector (G1362A Agilent). The eluent used was deionized HPLC water at a flow rate of 0.8 ml/min, and at a 20μl injection volume. Simultaneous analysis of organic acids, furfural and 5-HMF were performed using HPLC (Agilent 1200 series) with a Shimadzu VP-ODS column (250 mm × 4.6 mm I.D., Shimadzu, Kyoto, Japan) and a guard column (GVP-ODS, 10 mm × 4.6 mm ID). Two eluents were used. The first eluent was a sulfuric acid solution at pH 2.5 and the second eluent was methanol. Flow rate was maintained at 0.35 ml/min. The Diode Array Detector (G1315B Agilent) was set at 210 nm (UV spectra: 200 nm to 600 nm range) and the column temperature at 40 ºC. Sample injection volume was set at 20μl. Concentration of total phenolic compounds was measured as described by Alvira, et al [34]. Percent extraction (E) was calculated using Equation (1). All the values are the mean of three replicates with standard deviations.

Where Co and Ce (mg/L) are the initial and final concentrations of the compound in the aqueous phase, respectively
Statistical significance was detected by analysis of variance (ANOVA) and Tukey’s honest significant difference (HSD) test at a 95% confidence level using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Energy cane bagasse was composed of approximately 40.14 ± 0.16% cellulose, 24.23 ± 0.51% hemicellulose, 2.76 ± 0.04% arabinose, 24.41 ± 0.37% lignin (5.97 ± 0.16 acid soluble lignin, 18.44 ± 0.21 acid insoluble lignin), 3.76 ± 0.62 extractives, and 4.70 ± 0.04% ash (dry basis). Results were comparable to those published by others [2,36]. Liquid ammonia pretreatment removed 84.14% of the hemicellulose along with 60.23% of the lignin. Only a 9.01% glucan loss was observed. Approximately, 62.51% of the total solids were recovered after pretreatment.
Composition analysis of dilute ammonia pretreated energy cane bagasse hydrolysates post enzymatic digestion consisted of 37.63± 0.44 g/L glucose, 3.41 ± 0.84 g/L xylose, 2.05 ± 0.17 g/L total phenolic compounds, 1.75 ± 0.03 furfural, 0.81 ± 0.34 g/L 5-HMF, 3.66 ± 0.51 g/L formic acid, and 4.41± 0.12 acetic acid. Furan derivatives (furfural and 5-HMF), organic acids (e.g., formic acid, acetic acid) and phenolic compounds (e.g., cinnamic acid, vanilic acid, 4-hydroxybenzaldehyde, vanillin, synringaldehyde, syringic acid, cathecol) can be generated during harsh pretreatment conditions from the degradation of cellulose, hemicellulose and lignin [37,38]. However, the nature and concentration of these compounds can be different based on the composition of biomass, biomass type and severity of pretreatment applied [39]. Dilute ammonia pretreatment followed by enzymatic hydrolysis yielded comparable amounts of fermentable sugars with fewer amounts of non-sugar compounds than those reported for acid hydrolysis pretreated biomass due to its milder treatment conditions and less hemicellulose solubilization [40]. Chandel, et al. observed that dilute hydrochloric acid pretreated sugarcane bagasse resulted in fermentable sugars concentration of 30.29 g/L and generated 5.45 g/L acetic acid, 1.89 g/L furfural and 2.75 g/L total phenolic compounds [41].Similar observations were reported by Mateo, et al. in olive tree residue acid hydrolysates with the release of 25.23 g/L fermentable sugars and the formation of 1.67 g/L acetic acid, 0.22 g/L 5-HMF and 3.76 g/L total phenolic compounds [42].
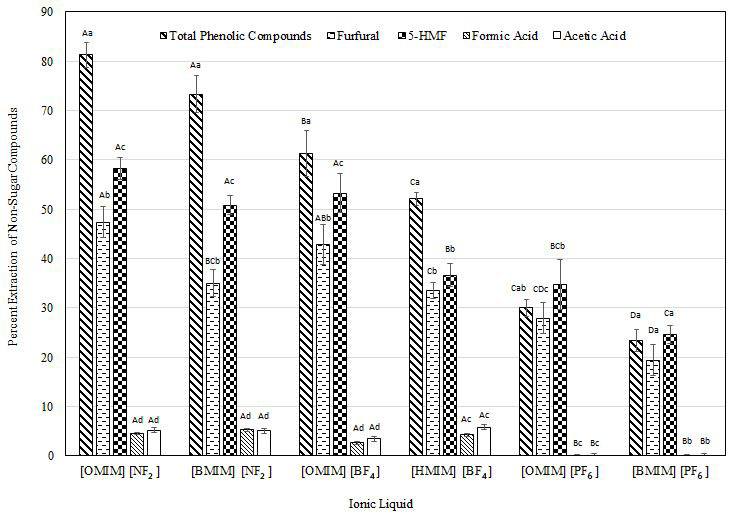
The presence of phenolic compounds (e.g., 4-hydroxybenzoic acid, phenol, coumaric acid, vanillin, catechol) in the hydrolysates can inhibit the growth of microbial cells during fermentation by interfering with the selective transportation of enzymes and substances through their cell membrane [18]. Phenolic compounds can also unproductively bind to the hydrolyzing enzymes. However, the effective extraction and recovery of these lignin-derived phenolic compounds can find potential applications in the food, pharmaceutical and cosmetic industries [43]. In this study, phenolic compounds were extracted by all six imidazolium-based ionic liquids (Figure 2). [OMIM][NF2] was the most effective with 81.30% extraction closely followed by [BMIM][NF2] with an extraction of 73.33%, which were significantly (p< 0.05) different than the other four ionic liquids. The least effective was [BMIM] [PF6 ] with a 23.36% extraction. Extractability of phenolic compounds using hydrophobic ionic liquids has been reported by others [35,44-46]. Archana, et al. assessed the removal of phenolic compounds using encapsulated room temperature ionic liquids and were able to remove 92.50% of the phenolic compounds from waste water at optimized conditions (4 hmixing time, 600 rpm mixing speed, 70 °C) [44]. Fan, et al. investigated the effect of 1-methyl-3-alkylimidazolium hexafluorophosphate [CnMIM] [PF6 ] (n = 4, 6, 8) and 1-methy-3-alkylimidazolium tetrafluoroborate [CnMIM][BF4] (n = 6, 8) for the extraction of phenolic compounds from waste water and observed that the nature of the ionic liquids as well as the chemical structure of the phenolic compounds can greatly affect extraction. Maximum extractability of phenolic compounds by ionic liquids takes place when they are in their un-dissociated form [35]. This promotes hydrogen bonding and hydrophobic interactions between the phenolic compounds and the ionic liquids [45]. The pH of our samples was close to 5 which are below the Pka of phenol (around 10). Most of the phenolic compounds have high Pka values (above 7) [46].This means that all the phenolic compounds were in their un-dissociated form favoring their extractability. Nosrati, et al. were able to extract 85% of phenolic compounds from waste water using 1-Butyl-3-methylimidazolium hydrogen sulfate [BMIM][HSO4] in combination with a hydrophobic polytetrafluorethylene membrane filter [45]. Khachatryan, et al. used [BMIM] [PF6 ] to extract phenolic compounds and reported considerable extractions after 10 min of mixing. However, phenol itself was not entirely extracted [47].
Hydrogen bonding capacity and hydrophobic interactions are the two main mechanisms of extraction between ionic liquids and phenols [48]. [NF2]−anion is the most hydrophobic anion with the strongest hydrogen bonding capacity from the ionic liquids evaluated [49,50]. Hence, highest extractibilitieswereobserved with [OMIM][NF2] and [BMIM][NF2]at 81.3% and 73.3%, respectively. Fan, et al. reported that when two ionic liquids had the same cation and alkyl chain, ionic liquids with anions of [BF4]− extracted more phenolic compounds than [PF6 ]− anions due to their stronger hydrogen bonding capacity [35]. Their observations agree with ours. A 61.30% extraction of phenolic compounds was observed with [OMIM][BF4]; whereas, only a 30% extraction was observed with [OMIM][PF6 ]. Hou, et al. reported the following order of anions for the extraction of phenolic compounds from oil using imidazolium-based ionic liquids: [Cl]– > [Br]– > [BF4]– > [PF6 ]–, with [BMIM][Cl] extracting 90% of the phenolic compounds [51]. They reported that the effect of anions on the extraction of phenolic compounds was more prominent than that of cations.
Extractibility of phenolic compounds can be affected by the type of anion present in the ionic liquid. However, the length of the alkyl chain also plays an important role in the extractibility of phenolic compounds. It was demonstrated that a longer alkyl chain of the cation present in the ionic liquid translates to better partitioning of the phenolic compounds into the ionic liquid as longer alkyl chains boost their hydrophilic interactions [44,45].We observed the same effect of alkyl chain length on the extractability of phenolic compounds from dilute ammonia pretreated energy cane bagasse hydrolysates. [OMIM]+ with an eight-carbon alkyl chain showed higher extractions (81.3%) as compared to [BMIM]+ which has a four-carbon alkyl chain (73.3%).
Furan derivatives (furfural and 5-HMF) can inhibit glycolysis through deactivation of the enzyme dehydrogenase thus interfering with cell replication [52]. Furfural is often present in larger amounts than 5-HMF and it can be more toxic to microorganisms [53]. These compounds, however, can have great applications in various industries. 5-HMF can be converted to levulinic acid, dimethylfuran, 2,5-furandicarboxylic acid, and dihydroxymethylfuran, which are building blocks in the manufacture of alternative fuels, polymers, foams, and polyesters [54]. Furfural has several applications as an additive in anti-acids, inks, fungicides, adhesives, and flavoring agents [55]. In this study, furfural and 5-HMF partitioned into ionic liquids at relatively lower amounts as compared to phenolic compounds (Figure 2). [OMIM][NF2] was the best ionic liquid for extracting furfural from the aqueous phase with an extraction of 47.44% closely followed by [OMIM][BF4] with an observed extraction of 42.80% (p> 0.05). [BMIM][PF6 ] showed the lowest furfural extractability at 19.47%.[OMIM][NF2] and [OMIM][BF4] worked best in extracting 5-HMF with 58.25% and 51.80% extractions, respectively, and were not significantly different (p>0.05) from [BMIM][NF2],which closely followed at 50.80% extraction. The lowest extraction was observed with [BMIM][PF6 ] at 24.40% (p<0.05). Limited information exists on the extraction of furfural and 5-HMF by ionic liquids. However, it has been suggested that the type of anion as well as the cation and its alkyl chain are important in partitioning furfural and 5-HMF into the ionic liquids. Pei, et al. investigated the effect of [BMIM] [PF6 ], [OMIM][PF6 ] and 1-hexyl-3-methylimidazolium hexafluorophosphate [HMIM][PF6 ] in extracting pure furfural and 5-HMF from an aqueous solution [55]. It was reported that [HMIM][PF6 ] worked best at a mixing ratio of 5:1 (aqueous solution to water) resulting in 76% furfural and 83% 5-HMF extractions. Furthermore, adding sodium chloride or sodium sulfate to the aqueous phase increased the extraction of furfural because of the competitive hydration of the salts with furfural.
The imidazolium-based ionic liquids evaluated in this study barely extracted the organic acids present in the hydrolysates, with observed extractions of less than 5% (Figure 2). However, if effectively extracted, organic acids could be used as potential platform chemicals to many bio-based products including silage and animal feed, food preservatives, catalysts, and plasticizers from formic acid and levulinic acid, and in the production of biodegradable polymers as in the case of acetic acid [54,56]. Our results agree with those published by Matsumoto, et al. They reported a poor extractability of organic acids (including acetic acid) from fermentation broths using imidazolium-based ionic liquids [57]. It was observed that the extractability of the organic acids was affected by the hydrophobicity of ionic liquids with an order of extractability reported as follow: [OMIM] [PF6 ] < [BMIM][PF6 ] < [HMIM][PF6 ]. McFarlane, et al. investigated the effect of nine different hydrophobic ionic liquids in the extraction of polar water pollutants including organic acids [58]. In agreement with our observations, it was reported that acetic acid did not partition into the ionic liquid phase regardless of the extraction criteria. Klasson, et al. investigated the effect of ionic liquids including [BMIM] [PF6 ], [BMIM][NF2] and [OMIM] [NF2] on the extraction of acetic, lactic and succinic acids from fermentation broths [59]. They reported the same failure of imidazolium-based ionic liquids to extract the organic acids regardless of the pH of the solution. The only promising results were observed with a sulfonate-anion ionic liquid (trihexyltetradecylphosphonium methanesulfonate) for the extraction of succinic acid. However, diluents such as nonanol and trioctylamine were used to improve the performance of the ionic liquid due to its high viscosity.
The high polarity of formic acid and acetic acid prevents them from properly partitioning into hydrophobic ionic liquids; however, they might partition well into hydrophilic ionic liquids. Lopez and Hestekin were able to effectively improve the extractability of organic acids from the water phase into hydrophilic ionic liquids1-ethyl-3-methylimidazolium trifluoromethanesulfonate ([EMIM][OTf]) and 1-butyl-3-methylimidazolium acetate ([BMIM][OAc]) using electrodialysis [60]. It was reported that ionic liquids showed different affinity towards organic acids and that this difference was prominently dictated by their types of anions. Similarly, Li, et al. was able to improve the extraction of butyric acid and its salts by using [EMIM][OTf] assisted with electrodialysis [61].
The alkyl chain of an ionic liquid affects its distribution ratio. It is shown that ionic liquids with higher distribution ratios are better solvents for the extraction of organic compounds. Furthermore, hydrophilicity of the compound in relation to the distribution ratio of the ionic liquid has an important role in the extractability of organic compounds [62]. Lateef, et al. were able to extract lactic acid from wine using 1-butyl-3-methylimidazolium bromide ([BMIM][Br]) at 80 ºC after 30 min of mixing at 800 rpm [63]. A recovery yield of 36% was achieved using diethyl ether. Oliveira et al. compared the extractability of lactic acid, malic acid and succinic acid using phosphonium-based ionic liquids ([P66614] [Cl], [P66614] [Dec], [P66614] [Phos]) [64]. They observed that the anion had a significant effect on the extractability of the organic acids. As for the first two ionic liquids with chlorine and decanoate anions ([P66614] [Cl] and [P66614] [Dec]), succinic acid showed the highest partition coefficient due to its lower hydrophilicity and tendency to leave water coupled with its smaller molecular size. However, lactic acid extraction was only improved when the anion of the ionic liquid was a phosphinate. They suggested that this was due to the hydrogen bonding of the phosphinate anion with the pendant hydroxyl group of lactic acid. The highest recovery of organic acids was 73% at optimum conditions.
Treatment of hydrolysates with imidazolium-based ionic liquids (5 min mixing) resulted in minimal sugar losses (<4%) (Table 1). However, sugar losses greater than 5% were observed after 10min, 15 min and 20 min of mixing (data not shown). [BMIM][NF2] resulted in losses of 3.80%, followed by [HMIM] [BF4] with 2.60% and by [OMIM] [NF2] with 1.83%. Sugar losses of less than 0.80% were observed with [OMIM] [BF4], [OMIM] [PF6 ] and [BMIM][PF6 ]. The order observed for the solubility of sugars in the ionic liquids matched the strength of the ionic liquids’ hydrogen bonding. [NF2]- has the strongest hydrogen bonds (3.40 kcal.mo–1) followed by [BF4]- (3.30 kcal•mol–1) and [PF6 ]- (2.40 kcal•mol1) [42]. Crosthwaite, et al. observed a similar trend in the affinity of these ionic liquids with alcohols [65]. These results are comparable to ours because like carbohydrates solubilization of alcohols happens through the hydrogen bonding of the ionic liquid with the solvent.
Low percentages in sugar losses are desirable as sugars are the main carbon source for microorganisms during fermentation. Therefore, keeping sugar losses to a minimum is a must during the removal and recovery of non-sugar compounds from hydrolysates. The results observed were as expected as the ionic liquids used in our experiments are hydrophobic in nature. For a carbohydrate to be soluble in an ionic liquid, a hydrogen bonding capacity between the anion of an ionic liquid and the hydroxyl group of a carbohydrate must take place [64]. Carneiro, et al. investigated the solubility of monomeric sugars in both hydrophobic and hydrophilic ionic liquids and observed that regardless of the hydrophobicity or hydrophilicity of the ionic liquid, solubility of sugars was as follows: fructose > xylose > glucose > galactose [66]. Based on our results, anions had a dominant effect on sugar losses as compared to cations. In ionic liquids containing the same anion, the ones with a shorter alkyl chain ([BMIM]+with a four-carbon alkyl chain)caused the most sugar losses due to their higher solubility. On the other hand, a cation with a longer alkyl chain ([HMIM]+ with a six-carbon alkyl chain or [OMIM]+ with an eight-carbon alkyl chain) has higher hydrophobicity and results in less amount of sugars being lost to solubilization [67].
[HMIM][BF4], [OMIM] [BF4], [BMIM][NF2] and [OMIM][NF2]extracted the most phenolic compounds, furfural and 5-HMF from hydrolysatesand were further assessed for their regeneration (Table 1). Regeneration of ionic liquids for organic acids were not conducted due to their poor extractability as previously reported. No detectable sugar losses were observed for any of these four ionic liquids after regeneration. All four ionic liquids extracted at least 93% phenolic compounds, furfural or 5-HMF after their first regeneration (second use). [HMIM][BF4] and [OMIM][BF4] retained 93% and 97% extract abilities for phenolic compounds, respectively, after their first regeneration (second use). After the second regeneration (third use), extraction of phenolic compounds decreased to 77% with [HMIM][BF4] and to 76% with [OMIM][BF4]. [OMIM][NF2] showed a 95% extraction for phenolic compounds after the first regeneration (second use) and 83% extraction after the second regeneration (third use). [BMIM][NF2] showed a 96% extraction for phenolic compounds after the first regeneration (second use) with extractions dropping to 74% after the second regeneration (third use). Archana, et al. used similar regeneration methods for the recovery of Cyanex – 923 when extracting phenolic compounds and recommended no more than two regenerations [44]. It was reported that extractions dropped from 96% (first regeneration) to 95% (second regeneration) and to a final 79% (third regeneration). The lower percent extractions observed in our study can be attributed to the differences in the extraction conditions used, such as pH and temperature. There is a wide variety of phenolic compounds with different Pka values in the hydrolysate, and the type and chemical composition of the phenolic compounds play a major role in their extractability by ionic liquids [46].
Extractions ranging from 92% to 94% for furfural and from 93% to 97% for 5-HMF were observed with [HMIM][BF4], [OMIM][BF4], [BMIM][NF2] and [OMIM][NF2] after their first regeneration (second use). After the second regeneration (third use), extractions of furan derivatives decreased to 60% with [HMIM][BF4], 71% with [OMIM][BF4], 73% with [BMIM][NF2], and 70% with [OMIM][NF2]. Our results indicate that no more than two regenerations are recommended for [OMIM][BF4], [HMIM][BF4], [BMIM][NF2] and [OMIM][NF2] when extracting phenolic compounds, furfural and 5-HMF from dilute ammonia pretreated energy cane bagasse enzymatic hydrolysates.
The presence of non-sugar compounds in hydrolysates, which nature and concentration are directly related to biomass composition and pretreatment conditions, can have a negative impact on downstream processes. The use of imidazolium-based ionic liquids as a method for their removal and recovery for use as building blocks to various chemicals in the food, pharmaceutical and polymer industries has potential. Of the six ionic liquids evaluated, [OMIM][BF4], [HMIM][BF4], [OMIM][NF2] and [BMIM][NF2] were most successful in the recovery of phenolic compounds, furfural and 5-HMF from dilute ammonia pretreated energy cane bagasse enzymatic hydrolysates, with organic acids failing to partition. No more than two regenerations of [OMIM][BF4], [HMIM][BF4], [OMIM][NF2] and [BMIM][NF2] are recommended for the extractability and recovery of phenolic compounds, furfural and 5-HMF from hydrolysates. However, the effectiveness of detoxification with ionic liquids depends not only on the nature of the ionic liquid but on the type of biomass, pretreatment and hydrolysis conditions used, and the chemical composition of hydrolysates.
USDA-NIFA Award No.: 2011-69005-30515z.