Role of Vitamin E and Rutin as Potent Inducers of Chlorpyrifos Degradation in the Blood of Treated Male Albino Rats
Determination of chlorpyrifos technical (CPF.T) or formulated (CPF.F) concentrations alone or in combination with vitamin E (Vit. E) or rutin (RT) (ameliorative agents) in the blood of treated male rats after 1, 4, 8 and 12 hrs from oral treatment. Gas chromatography (GC) analysis was done to determine CPF concentrations. Additionally, the ameliorative effects of Vit.E or RT, on the toxicity of CPF to rats were determined. Rats were divided into seven groups as follows; Control group, rats were given daily oral dose of corn oil only, CPF.T, CPF.T+RT, CPF.T+Vit.E, CPF.F, {(CPF.F) + RT}, {(CPF.F) + Vit.E}. Rats were treated daily with RT (50 mg/ kg b.wt.) or Vit.E (100 mg. kg-1 b.wt.) orally for 10 days, and treated with a single oral dose of CPF (10 mg. kg-1 b.wt.) at the 10th day. After oral administration of the last dose, blood was collected and analyzing by GC (ECD) to determine the concentrations of CPF after 1, 4, 8 and 12 hours from treatment with a single CPF oral dose. Results demonstrated that the concentrations of CPF were significantly decreased in the blood of rats pretreated with Vit.E or RT comparing with those receiving only single oral dose of CPF. Maximum concentration (C max) of CPF was obtained after 4 hrs of administration of CPF.F, CPF.F+ RT or CPF.F+ Vit.E as 2162.65, 1565.69 and 1489.43 ng/ml. Therefore, rutin and vitamin E may diminish the toxicity of this pesticide.
Keywords:Chlorpyrifos; Rutin; Vitamin E; Rats; Blood
Pesticides are ubiquitous in the environment and have positive environmental and public health impact. Moreover, their usage helps in improving human nutrition value. These pesticides have been used incidentally in large amounts and have also been largely involved in progressive pollution and health hazards. Human are potentially exposed to these pesticides either directly, as workers in agriculture, or indirectly, via food consumption. In addition, it is likely that a significant amount of these pesticides and their metabolites reach rivers and estuaries via run-off from farm land that are potentially toxic to wildlife [1]. Organophosphates pesticides (OPs) are occasionally used indiscriminately in large amounts causing environmental pollution [2-4]. Ops have an adverse effect on fertilization, liver function and central nervous system [5,6]. Recently, evidence of adverse health due to low-level exposure to OPs has begun to emerge [7]. Chlorpyrifos is widely used worldwide in agriculture and residential pest control [8]. Chlorpyrifos is effective against a broad spectrum of insect pests on a variety of crops like grain, cotton, fruit, nut, and vegetables [9]. Like other organophosphorous pesticides, its insecticidal action is due to the inhibition of acetyl cholinesterase, resulting in the accumulation of the neurotransmitter, acetylcholine, at nerve endings [10,11]. Vitamin E (alpha-tocopherol) is a fat soluble vitamin which regulates different oxidation processes in the body as it acts as a powerful antioxidant. Previous studies revealed that dietary intake of Vit.E can normalize the damaging effect of oxidative stress induced by oxygen free radicals [12-14]. A number of studies have concluded that Vit.E in combination with flavonoid synergistically inhibits oxidative damage both in vivo and in vitro [15-17]. In addition, it has been shown that alpha-tocopherol in combination with rutin and ascorbic acid synergistically inhibits lipid peroxidation in xanthine–xanthine oxidase system and human erythrocyte membranes [16]. Rutin (RT), is a well-known flavonoidal glycoside and set as an affective phenolic compound. RT has shown pharmacological benefits including anti-tumor, anti-inflammatory and hepato protective activities [18-20]. It is an antioxidant, comprised of the flavonol quercetin and the disaccharide rutinose. RT has been demonstrated to scavenge superoxide radicals. It also can chelate metal ions such as ferrous cations [21]. Moreover, RT has inhibitory effects against membrane lipid peroxidation and generation of reactive oxygen species (ROS) [22]. Hence, exposure to this insecticide may involve a large segment of the population, which includes agriculture workers and their families, those living in proximity to farms/orchards, and the general population who may be exposed through home application of pesticides or via residues on food [23,24]. Therefore the present study was undertaken to determine the effects of anti-oxidants (Vit.E or rutin) concentrations of chlorpyrifos on blood male rats treated with a single dose.
Chlorpyrifos technical [O, O-diethylO-3, 5, 6-trichloropyridin-2-ylphos-phorothioate] (purity >99.9%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Chlorpyrifos formulated (Pestban® 48 % EC) was purchased from Agrochem, Alwataneia Company, Alex., Egypt. Rutin a quercetin-3-rutinosid and sophorin or vitamin-P and n-hexane were procured in their highest grades available from Sigma-Aldrich (St. Louis, MO, USA). Pure water was deionized and double distilled with a Milli-Q-water purification system (Millipore). Vitamin E obtained from a local pharmacy. All other reagents used were of analytical grade.
Animals: Eighty-four male rats (Sprague Dawley) (weighing 150–170 g) were obtained from the Animal House of the Faculty of Medicine, Alexandria University, and Alexandria, Egypt. Animal handling and experimental design procedures were approved by the Research Ethical, Committee of the Faculty of Medicine, Alexandria University, Alexandria, Egypt and the protocol conform to the National Institutes of Health Guidelines. Animals were caged in groups given food and water ad-libitum. The animal room was maintained at 23 ± 2 °C and 40–60% relative humidity with 12h L: D cycles, the light cycle coinciding with the daylight hours. Rats were acclimatized for 2 weeks prior to the beginning of the experiment.
Dosages of each administered material were freshly prepared and adjusted weekly for body weight changes and given at approximately the same time each morning. Chlorpyrifos technical or formulated, rutin and vitamin E were administered at doses (10, 50 and 100 mg. kg-1 b.wt, respectively) according to the methods of [25,26]. The rats of check (control) group received an equivalent volume of corn oil (0.5 ml /rat). Animals were divided into 7 groups of rats while each group comprised 12 animals as following: Group 1, rats in this group served as control. Group 2, rats in this group were orally administered with the selective dose of chlorpyrifos technical (10 mg. kg-1 b.wt.) at the tenth day of the experiment [25].Group 3, Rats in this group were orally administered the selective dose of rutin (50 mg. kg-1 b.wt.) for 10 days, at the tenth day rats were administered a single dose of chlorpyrifos technical (10 mg. kg-1 b.wt.) [26]. Group 4, Rats in this group were orally administered the selective dose of Vitamin E (100 mg kg-1 b.wt.) for 10 days, at the 10th day rats were administered a single dose of chlorpyrifos technical (10 mg kg-1 b.wt.). Group 5, Rats in this group were orally administered the selective dose of chlorpyrifos formulation (10 mg kg-1 b.wt.) at the tenth day of the experiment [25]. Group 6, Rats in this group were orally administered the selective dose of rutin (50 mg. kg-1 b.wt.) for 10 days, at 10th day rats were administered a single dose of chlorpyrifos formulation. Group 7, Rats in this group were orally administered the selective dose of Vitamin E for 10 days, at the 10th day rats were administered a single dose of chlorpyrifos formulation [26].
Blood collection was done according to the method [27]. On the 10th day (after last doses) three rats of each group were scarified under ether anesthesia. Blood samples were withdrawn and collected in heparinized tubes at 1, 4, 8 and 12 hrs after oral administration of chlorpyrifos. No mortality was observed during the study.
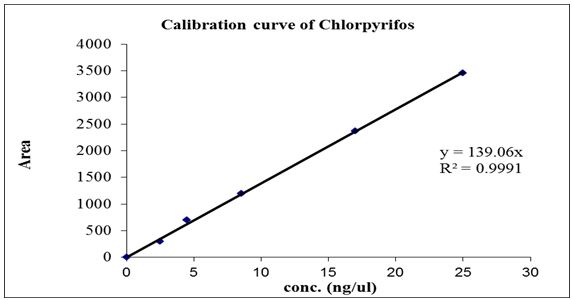
Assay of chlorpyrifos concentrations in male rat’s blood:According to the method of, 0.2 ml of whole blood was placed in Eppendorf tubes and 0.6 ml of n-hexane was added [25]. The samples were extracted by shaking for 10 min. followed by centrifugation at 13,000 rpm. Two μl of the hexane layer were analyzed by GC-ECD. Blood from untreated rats served as a blank matrix to prepare the calibration curve (Figure 1). The appropriate volumes of n-hexane solutions of standards were placed in the Eppendorf tubes and gently evaporated in a stream of air at room temperature. One ml of blood was then pipetted to the tubes and shaken for 30 min. The aliquots of spiked blood were processed as unknown and the calibration curve was based on the peak area of the analyses.
The analyses were performed in Environmental Chemistry & Natural Resources Center, Faculty of Agriculture, Cairo University, Giza, Egypt. The analyses were performed using a Hewlett Packard HP 6890 gas-chromatograph with electron capture detector (ECD). The column was an HP 5 (30 m x 0.320 mm x 0.25 μm film thicknesses). The on-column injector was operated at 270 °C and the detector at 300 °C. The temperature program for the column oven was as follows: 140 °C to 270 °C at 25 °C min-1 and then ramped at 30 °C to 300 °C with 8 min. hold. The carrier gas was nitrogen at a flow rate of 3 ml/min., and the injection volume was 1μl. standard for chromatography was done using chlorpyrifos technical.
The results were expressed as means ± S.E. All data were done with the Statistical Package for Social Sciences (SPSS Version 12.0 for Windows 7 operating system). The results were analyzed using one way analysis of variance (ANOVA) followed by Duncan’s test for comparison between different treatment groups. Statistical analysis was significantly at p < 0.05.
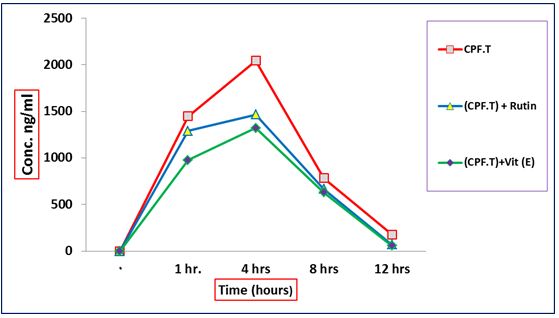
Concentrations of technical chlorpyrifos alone or in combination with Vit E or RT, in blood of male rats Data in indicated the amount of chlorpyrifos in the blood of male rats, after oral administration of {(CPF.T)}, {(CPF.T) + RT} and {(CPF.T) + Vit.E} after 1, 4, 8 and 12 hrs (Table 1) and (Figure 2). The initial concentrations of chlorpyrifos were 1448.37, 1290.16 and 976.41 ng/ml (at 1h.) after administration of {(CPF.T)}, {(CPF.T) + RT} and {(CPF.T) + Vit.E}, respectively. However the results indicate that the percent rates of loss to {(CPF.T)} was 10.9 and 32.58% in {(CPF.T) + RT} and {(CPF.T) + Vit.E}, respectively. The detected residue amounts of chlorpyrifos were elevated to be 2043.57, 1465.81 and 1319.96 ng/ml after 4 hrs of administration of {(CPF.T)}, {(CPF.T) + RT} and {(CPF.T) + Vit.E}, respectively. However, the concentrations of chlorpyrifos were 784.55, 667.12 and 628.51 ng /ml at 8 hrs after administration of {(CPF.T)}, {(CPF.T) + RT} and {(CPF.T) + Vit.E}, respectively. After 12 hrs the concentrations of chlorpyrifos decreased to be 176.91, 70.47 and 56.09 ng/ml for {(CPF.T)}, {(CPF.T) + RT} and {(CPF.T) + Vit.E} groups, respectively.
Values are mean of 3 replicates and given as mean ± SE. Different letters in the same column indicate significant differences according to (CPF.T).
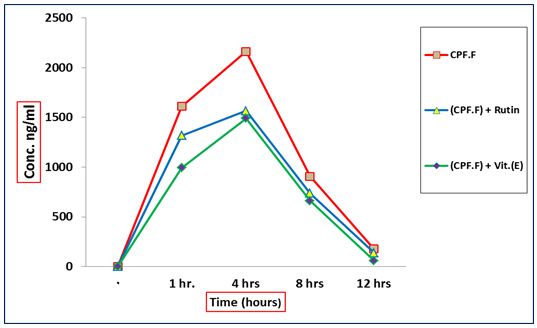
Concentrations of formulation chlorpyrifos alone or in combination with Vit.E or RT, in blood of male rats Data in indicated the amount of chlorpyrifos in the blood of male rats were 1610.82, 1318.57 and 994.18 ng/ml after 1hr of administration of {(CPF.F)}, {(CPF.F) + RT} or {(CPF.F) + Vit.E}, respectively (Table 2) and (Figure 3). On the other hand, Maximum concentration (C max) of CPF was obtained after 4 hrs of administration of {(CPF.F)}, {(CPF.F) + RT} or {(CPF.F) + Vit.E} as 2162.65, 1565.69 and 1489.43 ng/ml. However, the concentrations of chlorpyrifos were 906.87, 737.02 and 660.51 ng /ml at 8 hrs after administration of {(CPF.F)}, {(CPF.F) + RT} or {(CPF.F) + Vit.E}, respectively. After 12 hrs the concentrations of chlorpyrifos decreased to be 177.44, 138.73 and 60.45 ng/ml for {(CPF.F)}, {(CPF.F) + RT}, {(CPF.F) + Vit. E} groups, respectively.
The concentrations of CPF detected in groups treated with CPF.F were higher than those detected in groups treated with CPF.T in all treatments. While,Vit.E was more potent inducer in CPF degradation than RT.
Values are mean of 3 replicates and given as mean ± SE. Different letters in the same column indicate significant differences according to (CPF.F).
Chlorpyrifos is the active ingredient in many commercial insecticide formulations [28]. However, the toxicity associated with chlorpyrifos is due to the oxon that inhibits acetyl cholinesterase, whereas neither chlorpyrifos itself nor its metabolite could be able to inhibits acetyl cholinesterase activity or that of other serine-dependent esterases or proteases [29,30]. Several analytical methods have been used for the identification and quantization of this pesticide and its metabolite when applied alone in crop protection. Among these methods were high-performance liquid chromatography (HPLC), HPLC-MS, Gas chromatography (GC), and GC–MS [31-37]. While, the most analytical methods have been determined the concentration of chlorpyrifos in food, air, and in offices; no sufficient published studies have concerning the influence of a single dose of chlorpyrifos and its concentration after a matrix of time in rats, blood, for this reason, it is very difficult to compare our results with those reported by other authors. Administration of chlorpyrifos to rats induced oxidative damage and caused injury to liver, kidney and endocrines of male rats [38-42]. Our data indicated that, when a single dose of chlorpyrifos was administered, C max was obtained after 4 hrs of administration in all treatments. These results were in agreement with [25]. The assessment of the influence of Vit.E and RT on the CPF concentrations in rats blood, revealed their role in the CPF degradation and this was in agreement with [43]. {(CPF.T) + RT}, {(CPF.T) + Vit.E}, {(CPF.F) + RT}, {(CPF.F) + Vit.E} groups confirm these findings (Tables 1 and 2). The current study illustrated the contributory roles of Vit.E and rutin on the CPF concentrations in rat’s blood. In fact, xenobiotic-phytochemical interactions are well established. Phytochemicals have the potential to both elevate and suppress the activity of cytochromes (phase I detoxification enzymes) involved in the biotransformation of xenobiotics [44]. On the other hand, pesticide mediated toxicity involves excessive production of reactive oxygen species (ROS), leading to alterations in the cellular antioxidant defense system and consequently affecting susceptibility to oxidative stress. Alterations in non-enzymatic antioxidants, like gluthatione and total thiol, as well as scavenging enzymes, such as superoxide dismutase and catalase have been reported [44-50]. In addition to other low molecular weight substances, the non-enzymatic antioxidant systems includes vitamins, like vitamin C and E, suggesting that imbalance caused by oxidative stress can be replenished by dietary antioxidant intake. With respect to that assumption, the findings by have demonstrated the lipid peroxidation in rats. Apart from vitamins, non-vitamin substances, such are polyphenols, can also reinforce antioxidant defense [49,50]. Khan SM, RC Sobti and L Kataria (2005) Reported that pretreatment with the polyphenolic fraction of black tea increased reduced glutathione and total thiol antioxidant reserves, as well as the activities of the antioxidant enzymes, glutathione-peroxidase, glutathione S-transferase and glutathione reductase after exposure to pesticides [51]. Additionally, these enzymes are important components of the phase II detoxification metabolism and thus, polyphenol treatment promotes the excretion of xenobiotic from the body. Data of this work indicated that rats treated with rutin as co-treatment has significantly decrease chlorpyrifos concentration in rats blood in a time dependent manner comparing with those treated with a single oral dose of chlorpyrifos. Our finding was in agreement with who reported that rutin has effectively reversed the biochemical, behavioral, and neurochemical changes in rat treated with haloperidol and improved the antioxidants enzymes system in human [52]. This work results indicated that pretreatment or supplementary of vitamin E significantly decreased the concentration of chlorpyrifos in rats blood comparing with the un supplementary groups, this results may contributed to the fact that Vitamin E is a fat soluble vitamin which regulates different oxidation processes in the body as it acts as a powerful antioxidant. Previous studies revealed that dietary intake of vitamin E can normalize the damaging effect of oxidative stress induced by oxygen free radicals results from organophosphate compounds [12-14].
Data obtained in this study are particularly important, emphasizing the roles of rutin and Vitamin E as potent inducers of chlorpyrifos degradation. Rutin and Vitamin E have potential to lower the burden of the toxic effects of chlorpyrifos in animals. These findings can support the design of intervention strategies that deal with diminishing of xenobiotics toxicity. Therefore, the administration of both rutin and vitamin E may be of value to farmers and other workers who are frequently exposed to chlorpyrifos.