Screening of Different Chickpea Varieties for their Sensitiveness and Tolerance to Cadmium and/or Salt Stress
This study demonstrates that soil amended with NaCl (100mM) and/or Cd (50 μM) significantly altered the physiological functions of all the varieties of chickpea in the concentration dependent manner. Moreover, the combination of NaCl and Cd caused maximum toxicity reflected through increased H2O2 content, lipid peroxidation and electrolyte leakage in the leaves at the sampling stage. Variety PDG-4 showed maximum tolerance against toxic effects of NaCl and/or Cd and var. GNG-1581 established as sensitive variety. The order of damage for PSII under NaCl and/or Cd stress in the varieties was in the order of GNG-1581 > SKU-C-23311 > C-350 > PBG-5 > PDG-4. Form the study it can be suggested that variety PDG-4 could be adopted in the area where there is problem of NaCl and/or Cd toxicity in the soil and variety GNG-1581 could be exploited as phyto-marker for assessing the toxicity of NaCl and/or Cd in the soil by the farmers.
Keywords: Cadmium; H2O2 Content; Lipid Peroxidation; Salt Stress; Photosynthetic Attributes
With continuous use of anthropogenic activities like industrialization, utilization of pesticides and fertilizers, undesirable heavy metals have been discharged into the rivers and lakes leading to the pollution in the last few decades [1]. These deposited heavy metals greatly impair the plant growth and development [2]. These heavy metals later enter into the food chain putting animals and humans at risk. Like other heavy metals, cadmium (Cd) is a widespread pollutant which not only interferes in number of plant processes like photosynthesis, nutritional imbalances or respiration, it also causes the oxidative stress by the generation of reactive oxygen species (ROS) which disturb the antioxidant system of plants [3,4]. Moreover, Cd also inhibits the activities of the enzymes of photosynthetic machinery and metabolism of nitrogen, sulfur and carbohydrates [5].
Like Cd stress, salt stress is also the major factor limiting the growth and development of the plants [6]. It is increasing day by day either from natural weathering of saline rocks or from anthropogenic activities such as poor irrigation practices, application of chemical fertilizers, deforestation and overgrazing etc. Several salts such as NaCl, MgCl2, KCl, Na2SO4, MgSO4, CaSO4 and Na2CO3 dissolved in soil cause salinity stress among which NaCl is major salt contributing towards salinity stress [7]. High salinity stress is detrimental to plant growth and productivity causing considerable losses to yield of economically important crops by reduction in the enzymatic activities and the photosynthetic rate, thereby threatening sustainable agriculture [8]. Photosynthesis is process severely affected during salt stress through decrease in chlorophyll content, inactivation of rubisco enzyme and stomatal closure which brings about decrease in CO2 pressure [9]. However, lipid peroxidation, H2O2 content has been reported to be increased under salinity stress [10]. Salt stress has also been reported to alter the nitrogen metabolism in the leguminous plants by affecting its various enzymes [11]. Moreover, the toxic salts accumulated in the leaf apoplasm cause dehydration and turgor loss that lead to death of the cells and tissues.
It has been reported that salinity in the soil significantly enhances the Cd availability in the soil and promotes its uptake in the plants [12]. The mechanism of the salinity and Cd is not fully understood but it is postulated that Cd-Cl complex is responsible for the Cd uptake in the plants. Both salt and Cd stresses pose severe threats by inducing physiological dysfunctions limiting plant growth and development. Much work has been carried out on the Cd or salt stress individually but there is limited literature available on the combined effect of Cd and NaCl stress on plant growth and development. Moreover, it is the need of the hour to select the plant genotypes which have high tolerance to both Cd and salinity stress to counteract the adverse effects on growth and yield of the plants. Therefore, the present work was carried out to screen the tolerant and sensitive varieties of Cicer arietinum L. under Cd and/or NaCl stress based on photosynthesis and fluorescence in the plant leaves.
The uniform and healthy-looking grains of five different varieties (PDG-4, PBG-5, C-350, SKU-C-23311 and GNG-1581) of Cicer arietinum L. were obtained from Indian Institute of Pulses Research, Kanpur. The experiment was conducted with 100 earthen pots (6 inch in diameter) in a way where each treatment had five replicates and three plants were maintained in each pot, arranged under simple randomized block design. The seeds of these five different varieties were surface-sterilized with 0.01% mercuric chloride solution for 2 min followed by the repeated washing with double distilled water (DDW) to remove the adhered mercuric chloride particles on the seed surface. These surface sterilized seeds were then sown in the earthen pots filled with soil and sand (3:1) and then mixed with farmyard manure (9:1). These pots were stacked in Kashmir University Botanic Garden (KUBG) under natural environmental conditions with an average day/night temperature as 24 °C/11 °C. These pots were irrigated at an interval of two days. All the 100 pots were divided into 5 groups with 4 treatments per group. These groups represented 5 different varieties (PDG-4, PBG-5, C-350, SKU-C-23311, and GNG-1581) of chickpea. The 15-day old seedlings were treated with Cd (50μM) and/or NaCl (100mM).
The plants were then harvested after 45 days to assess various parameters:
Net Photosynthetic Rate and its Related Attributes: Stomatal conductance, internal CO2 concentration, transpiration rate were measured by LI-COR 6400; Lincoln, NE, USA (IRGA-Portable Photosynthesis System)
Maximum Quantum yield of PSII Fv/Fm: It was measured by LI-COR leaf Flourometer (LI-COR 6400-40)
Total Chlorophyll Content: Total Chlorophyll Content was measured by Lichtenthaler [13]
Lipid Peroxidation: Lipid Peroxidation was determined by the method proposed by Hodges, et al. [14]
H2O2 content: H2O2 content was determined by the method proposed by and Jana and Choudhari [15]
Electrolyte leakage: Electrolyte leakage in leaves was determined by Sullivan and Ross [16]
Membrane stability index: Membrane stability index was measured by the method given by Sairam [17]
Proline Content: Proline Content in leaves was determined by adopting the method of Bates, et al. [18]
Statistical Analysis: The data was analyzed for variance by two-way ANOVA by using SPSS® (SPSS ver. 17, Chicago, United States). Mean values were tested by least significant difference at 0.05% probability level.
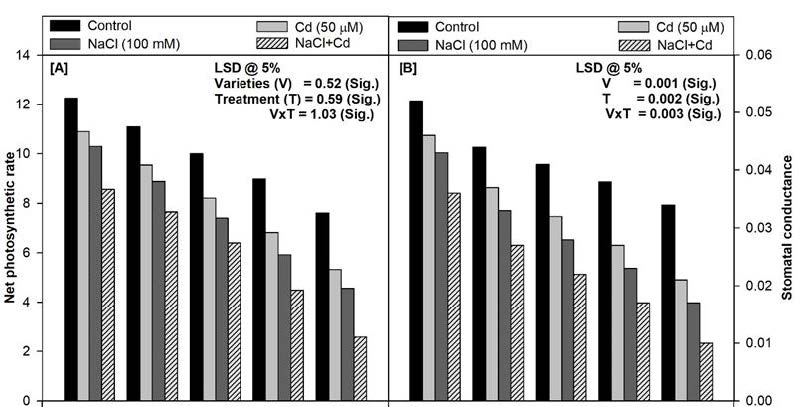
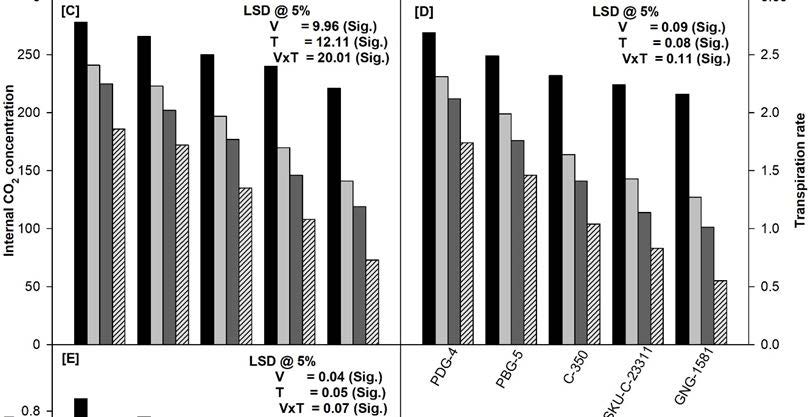
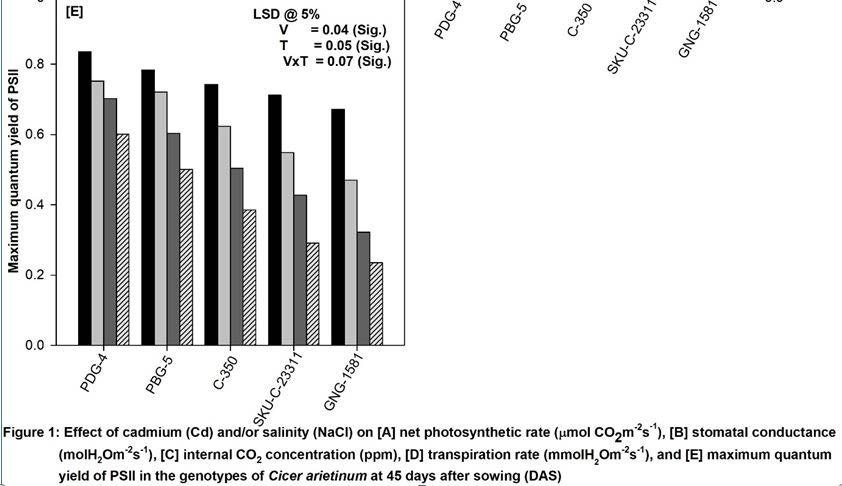
The data presented in Figure 1 clearly shows a significant decrease in net photosynthetic rate (Pn), stomatal conductance (gs), internal CO2 concentration (Ci), transpiration rate (E) and maximum quantum yield of PSII (Fv/Fm) of all the varieties tested at 45 days. Moreover, it was observed that variety PDG-4 showed highest rate of photosynthesis where as GNG-1581 showed lowest as compared to other varieties. The maximum reduction in the above parameters was found to be in GNG-1581 irrespective of stress treatments. The soil applied Cd (50 μM) and/or NaCl (100 mM) significantly decreased the above parameters in comparison to control plants. Moreover, the combination of NaCl and Cd proved deleterious and caused maximum damage to all the photosynthetic attributes in all the varieties. In terms of percentage, the decrease in Pn was 30%, 31%, 36%, 50%, 66%, Gs 29%, 37%, 45%, 56%, 70%; Ci 33%, 35%, 46%, 55%, 68%; E 35%, 41%, 55%, 63%, 75%; and Fv/Fm 28%, 36%, 48%, 59%, 65%; compared to their respective controls in PDG-4, PBG-5, C-350, SKU-C-23311 and GNG-1581 respectively at 45 days of growth (Figure 1).
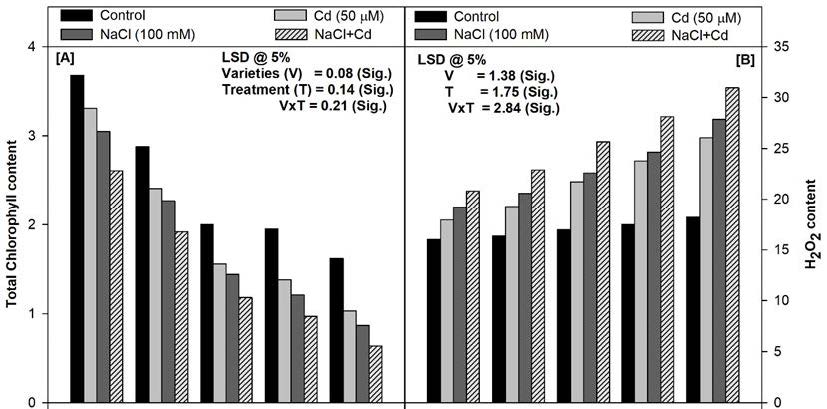
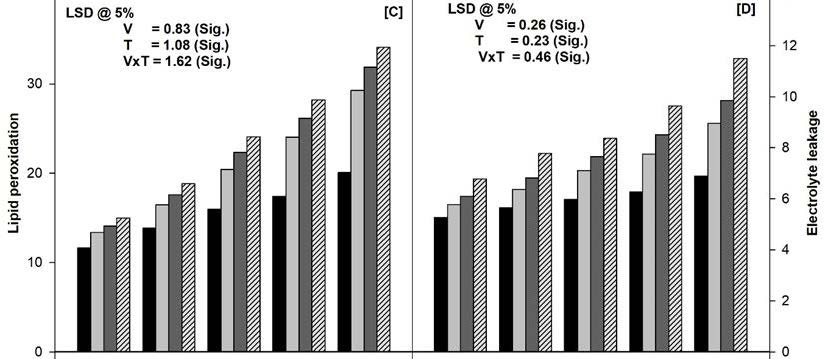
The NaCl and/or Cd present in the soil decreased the total chlorophyll content in all the varieties tested at 45 days of growth (Figure 2A). The combination of NaCl (100 mM) and Cd (50 μM) proved most deleterious and lowered the total chlorophyll content in var. PDG-4 (27%), PBG-5 (33%), C-350 (41%), SKU-C-23311 (50%), and GNG-1581 (60%) in comparison to their respective controls at 45 days of growth. Moreover, var. PDG-4 and GNG-1581 showed maximum and minimum tolerance respectively, against all the level of stresses (Figure 2A).
Figure 2B and 2c showed that both H2O2 content and LPO in the leaves increased significantly with the increasing level of stresses at 45 days of growth in all the varieties. The NaCl and Cd individually showed similar responses in all the varieties. The combination of NaCl and Cd showed maximum increase in the H2O2 content and LPO in comparison to the untreated control plants at the selected sampling stage. The maximum increase of H2O2 content and LPO was in the order of GNG-1581 > SKU-C-23311 > C-350 > PBG-5 > PDG-4 grown in the soil amended with the combination of NaCl and Cd at 45 days of growth (Figure 2B and 2C).
The presence of NaCl and/or Cd in the soil caused a significant increase in the electrolyte leakage in the leaves of all varieties compared with their respective control plants at the selected sampling stage (Figure 2D). The variety PDG-4 grown in soil devoid of NaCl and/or Cd i.e. control plants showed minimum values for electrolyte leakage in comparison to NaCl and/or Cd treated soil at 45 days of growth. However, electrolyte leakage increased in proportion to levels of NaCl and/or Cd whereas, NaCl (100 mM) and Cd (50 μM) in combination generated maximum values for electrolyte leakage in all the varieties. Variety PDG-4 showed maximum tolerance against NaCl and/or Cd stress.
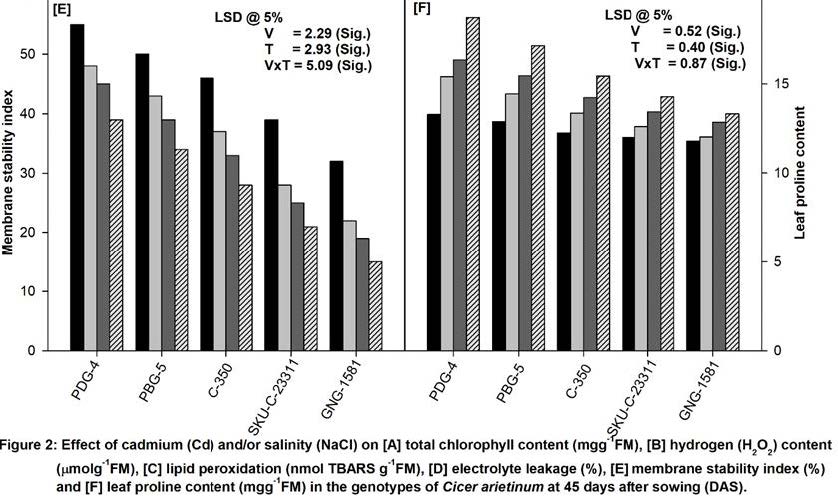
As depicted in the Figure 2E, the MSI decreased in the plants grown in the soil amended with NaCl and/or Cd at 45 days of growth. NaCl and Cd alone significantly decreased the MSI in all the varieties. Moreover, the NaCl and Cd in combination showed maximum decrease in MSI in all the varieties and in terms of percentage the decrease was 28% (PDG-4), 32% (PBG-5), 39% (C-350), 45% (SKU-C-23311) and 51% (GNG-1581) compared to their respective control plants (Figure 2E).
The data depicted in Figure 2F revealed that the leaves of plants grown in NaCl (100 mM) and/or Cd (50 mM) treated soil showed a significant accumulation of proline in all the varieties at the 45 days of the growth. Maximum proline content in all the varieties was noted under combination of NaCl (100 mM) and Cd (50 mM) stress and the pattern of increasing accumulation was in order of PDG-4> PBG-5 > C-350 > SKU-C-23311> GNG-1581 at the sampled stage.
Photosynthesis is said to be the backbone of the plants. Any change occurs in response to any external stimuli therefore it can be used as a good stress marker. In the present study, a clear-cut variability was found among the varieties of Cicer arietinum L. in response to the NaCl and/or Cd stress in terms of the net photosynthetic rate and its related attributes (Figure 1A-D). The order of the different varieties of chickpea regarding their photosynthetic performance is PDG-4>PBG-4>C-350>SKU-C-23211>GNG-1581. Both salinity and Cd decreases the partial pressure of CO2 in the stroma bringing about the closure of stomata that becomes the direct cause for the loss of gs, Ci and E as observed in the present study [19]. Moreover, these stresses damage the photosynthetic machinery at multiple levels such as pigment biosynthesis (Figure 2A), stomatal functioning and gaseous exchange, structure and function of thylakoid membrane, electron transport and enzyme activities which in turn lead to the decreased rate of photosynthesis ( Figure 1A) [20]. Moreover, low Pn values, under salt stress have been positively related to the observed decrease in gs and Ci [21]. Similar observations have also been made by other workers [11,19,22,23].
Cadmium and/or NaCl present in the soil significantly decreased the maximum quantum yield of PSII in all the five varieties of chickpea at 45d-stage of growth through damage in PSII electron transport system (Figure 1E). Both Cd and NaCl block the electron transfer from QA (primary acceptor plastoquinine) to QB (secondary acceptor plastoquinone) at the acceptor side of PSII leading to decrease in the Fv/Fm values [24]. Moreover, NaCl stress generates the physiological drought that increases the turnover of D2 protein of PSII leading to its decreased quantum yield. Our work is further corroborated by the research work done in wheat, mustard and tomato under different stress regimes [19,22,24].
Leaf chlorophyll content is an important physiological parameter extensively used as an indicator of chloroplast development and photosynthesis in the plants. The chlorophyll content has been reported to get reduced under various stresses depending upon the duration and degree of the stress [25]. In the present study, NaCl and/or Cd present in the soil decreased the total chlorophyll content either through its biosynthesis inhibition or by the acceleration of its degradation by the chlorophyllase enzyme [26]. Moreover, the light harvesting ability of chlorophyll is affected by replacing its central atom i.e. Mg, with Cd which is the important damage mechanism in the plants under heavy metal stress [27].
The heavy metal (Cd) and salt (NaCl) stresses reduce the membrane stability (reflected as decrease in the membrane stability index) in various crops leading to the leakage of essential ions reflected as electrolyte leakage in the present study [28]. Reduced membrane stability index in the present study may be the result of the peroxidation of membrane lipids under Cd and/or NaCl stress. Lipid peroxidation is accepted as an important criterion for assessing the magnitude of the oxidative stress. Increase in lipid peroxidation under Cd and/or NaCl stress has also been reported in soybean plants [29]. Moreover, Cd and/or NaCl caused increase in the H2O2 production in various varieties of chickpea in the present study. H2O2 is produced in the cells not only under normal conditions but also by the oxidative stress generated by drought, heavy metal, salt chilling stresses etc. [30]. Due to stomatal closure and low availability of CO2 (as observed in the present study as Ci) under Cd and/or NaCl stress, oxygenation of Ribulose-1,5-bisphosphate (RuBP) is favored and thus photorespiration is enhanced which leads to more than 70% of the H2O2 production [31].
In the present study, the proline content increased to prevent the oxidative damage due to physiological drought generated by Cd and/or NaCl and to boost the resistance capacity of the plants to overcome the inhibitory effects of Cd/or NaCl. Moreover, the activity of enzymes related to proline synthesis is up regulated and that of catabolizing enzymes is lowered under stress conditions [32]. The order of the different varieties of chickpea regarding the increase in proline content is PDG-4>PBG-4>C-350>SKU-C-23211>GNG-1581 indicating that the variety PDG-4 is efficient enough to adapt to the contaminated environment by developing the antioxidant defense mechanism followed by PBG-4. Similar observations have also been made in mustard [33].
Out of five varieties tested, PDG-4 and GNG-1581 established as NaCl and/or Cd tolerant and NaCl and/or Cd sensitive variety, respectively and it is speculated that lipid peroxidation, accumulation of H2O2 and electrolyte leakage is responsible for the deleterious effect of NaCl and/or Cd stress. In addition to this, variety PDG-4 showed maximum accumulation of osmolyte (proline) and as such has the ability to recover more effectively at later stages of growth under combination of NaCl and Cd stress. It can be concluded that the variety PDG-4 can be adopted in the area where there is problem of NaCl and/or Cd toxicity in the soil and variety GNG-1581 could be exploited as phyto-marker for assessing the toxicity of NaCl and/or Cd in the soil by the farmers.
Dr. Arif Shafi Wani thanks Science and Engineering Research Board (SERB), New Delhi, India for providing fellowship under the Young Scientist (SB/YS/LS-389/2013) Program.