Severe Residual Tricuspid Regurgitation with a Flail Leaflet Following Endovascular Pacing Wire Removal
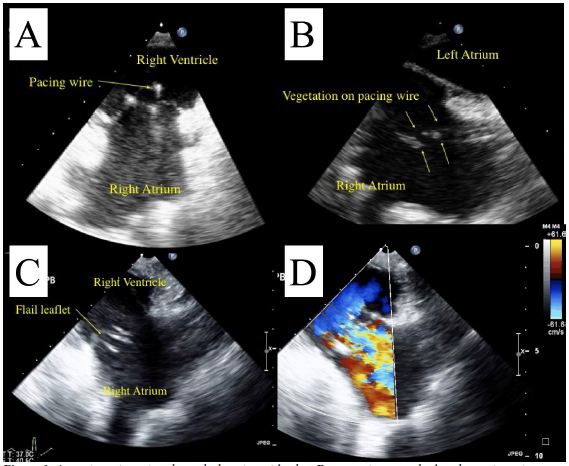
A 71-year-old man with a dual chamber pacemaker presented for a pacemaker lead extraction under general anesthesia. Two months prior to this admission, he had pyrexia and chills and was admitted to our hospital. Six separate blood cultures grew group G streptococcus, and transesophageal echocardiography revealed a mobile mass presumed to be a vegetation (1.4x8.4 cm) on the RV lead. Though the initial surgical plan was to extract the pacing lead as well as the generater under fluoroscopy with CPB on standby, it was impossible to safely remove the lead because it was so adherent to the RVOT. The surgical team decided to convert to the open heart surgery. The removal of this lead resulted in tears to the RA wall, SVC and the left subclavian vein with significant blood loss, which required blood transfusion. The post-CPB TEE showed severe tricuspid regurgitation due to flail anterior leaflet. We are unaware of guidelines or indications for open heart pacing lead extraction. The decision to replace a valve in a patient with ongoing sepsis is controversial and current recommendations are not suported by strong evidence. Further research is warranted to investigate the indication and timing of valve replacement surgery in a patient with tricuspid endocarditis.
Keywords: Venous thromboembolism (VTE); Deep Vein Thrombosis (DVT); Inferior Vena Cava (IVC); Pulmonary Embolism (PE); Transthoracic Echocardiography (TTE)
The pacing wire removal procedure is commonly performed in the catheter laboratory. In cases of old pacing wires (one year or longer), most frequently the procedure is performed in the operating room as rates of intraprocedural perforation and massive bleeding increase. The authors present a case of residual tricuspid regurgitation following removal of old pacing wires. The decision to replace a valve in a patient with ongoing sepsis is controversial and current recommendations are not suported by strong evidence. In this case, after a long discussion, the surgeons did not replace the tricuspid valve for fear of exacerbating active endocarditis. At the 2 month follow-up visit, the patient still remains asymptomatic and blood cultures came back negative. Further research is warranted to investigate the indication of valve replacement in a patient with tricuspid endocarditis.
A written informed consent for publication was obtained from the patient. A 71-year-old man with a dual chamber pacemaker presented for a pace maker lead extraction under general anesthesia. The dual chamber pacemaker was implanted in 1990 due to sick sinus syndrome, followed by a generator change in 1998 and 2004, and additional pacing wire insertions due to increased thresholds in 2014. Two months prior to this admission, he had pyrexia and chills and was admitted to our hospital. Six separate blood cultures grew group G streptococcus, on and transesophageal echocardiography revealed a mobile mass presumed to be a vegetation (1.4x8.4 cm) on the RV lead. He was found to have 2 leads in RA and 2 leads in RV. No valvular involvement was observed. He was treated with ceftriaxone 2 grams per day and pacemaker removal was planned. Prior to surgery, his blood pressure was 95/68 mmHg, heart rate of 90 (sinus rhythm), and he was not pacemaker dependent.
Initial TEE showed a mobile mass (1.4x8.4 cm) adherent to the atrial side of the RV lead (Video, Figure 1). No obvious involvement of valvular structures was observed.
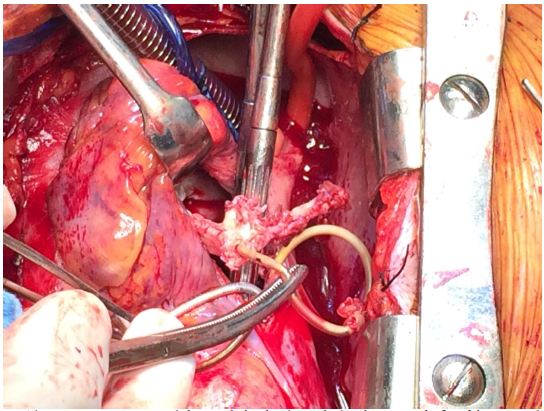
Though the initial surgical plan was to extract the pacing lead as well as the generator under fluoroscopy with CPB on standby, it was impossible to safely remove the lead because it was so adherent to the RVOT, and the surgical team decided it safer to convert to the open heart surgery. Intraoperatively, the RV leads were extremely adherent to the RV. One of the RV leads was stuck to the anterior leaflet of the tricuspid valve and there was evidence of significant destruction of the subvalvular apparatus of the anterior leaflet of the tricuspid valve. The surgeons observed that the chords to the anterior leaflet had been excised along with the vegetations and they pre-emptively attempted to repair the valve. Despite Gore-tex sutures to reconstruct the cords, there was significant residual tricuspid regurgitation (Figure 2). The RA lead was stuck to the RA wall, SVC wall and the left subclavian vein wall as well. The removal of this lead resulted in tears to the RA wall, SVC and the left subclavian vein with significant blood loss which required blood transfusion. Considering the significant blood loss, and evidence of bacteremia and likely sepsis, the surgical team decided against replacing or further attempts at repairing the tricupsid valve in order not to further complicate the procedure or provide foci for endocarditis, and a consensus decision was made to accept the severe residual tricupsid regurgitation.
The total CPB time was 73 minutes.
The post-CPB TEE showed severe tricuspid regurgitation due to flail anterior leaflet (Video). The RV was severely enlarged and the LV was compressed throughout the cardiac cycle, consistent with high pulmonary artery pressure.
The post operative course was uneventful and the patient was extubated on POD 1 and discharged from the ICU. At 2 month follow-up visit, he was found to be asymptomatic, and the blood culture was negative.
Right sided endocarditis is commonly observed in patients with pacemaker leads, IV drug users and immunocompromised patients. Recently, the incidence of pacemaker device infection has been increasing due to an a ging population with multiple co-morbidities including diabetes, heart failure and renal insufficiency. According to Greenspon, et al. vegetation size is a major determinant of mortality and patients with larger vegetations had higher mortality rates [1]. Usually vegetations are seen attached to the pacing leads or the valvular structures. In cases of valvular vegetations, valve replacement procedure is the standard of care. In cases of vegetations on the pacing leads, laser extraction of the pacing wire suffices in most cases. However, when pacing leads have been in situ for prolonged periods, and abundant scar tissue is seen around the insertion site, percutaneous extraction can be extremely difficult with increased risk of ventricular perforation. In such circumstances, sternotomy/thoracotomy may berequired for safe extraction. Regardless of the extraction technique, post-operative tricuspid regurgitation is commonly observed. Franceschi, et al. prospectively reviewed 208 patients undergoing pacing wire removal procedures and showed that moderate or severe post-removal tricuspid regurgitation was observed in 2.4% and 6.7%, respectively [2]. They also showed that 10.5% of those with post-removal tricuspid regurgitation died due to heart failure post-operatively. The mortality rate was higher in patients with residual TR compared with those without residual TR. In conclusion, they revealed that the use of laser sheath and lasso technique is associated with post-removal tricuspid regurgitation. Yasser Rodriguez, et al. prospectively studied 173 patients undergoing endovascular pacing lead extraction [3]. They showed that the most common indication was infection and all of their cases were done with an endovascular approach. The severity of TR in these patients was unchanged post procedure. Conversion to cardiac surgery is a rare event and no literature clearly defines the indications for elective open heart extraction. There are two important considerations in this case.
Firstly, whether or when to replace a valve in a patient with sepsis or endocarditis is controversial. There are no hard and fast established guidelines regarding the timing of surgery. This is due to the diversity of patients with infective endocarditis and the heterogeneity of patient morbidities [4]. Our patient started showing symptoms of sepsis three months prior to surgery and had been on antibiotics since he presented. Considering the risk of re-operation, valve replacement could have been justified.
Secondly, although we started this case with an endovascular approach, as soon as it became evident that this approach was unlikely to succeed, we aborted the endovascular approach and elected to perform sternotomy. According to Brunner, et al., in-hospital mortality among patients that required emergency surgical or endovascular intervention was 36% [5]. Emergency endovascular intervention included the deployment of intravascular devices. Considering the high mortality in emergency situations, early conversion to surgical extraction could be a wise option in the face of difficult endovascular extraction. We suggest that the succesful outcome in this patient was in no small measure due to timely abandonment of the endovascular approach and a controlled conversion to sternotomy and CPB. According to Prendergast, et al., tricuspid valvectomy without use of a prosthetic can be effective in extreme cases, however, this may be associated with severe postoperative right-sided heart failure [4]. We are unaware of any established guidelines supported by strong evidence to support valvectomy or valve repalcement in these circumstances. As such, we suggest individualized care needs to be applied to each patient in similar circumstances, and the risks of prosthesis endocarditis and prolonged CPB need to be carefully weighed against the consequences of severe TR.
In conclusion, we report a case of pace maker extraction in a patient with active endocarditis and echocardiographic evidence of vegetation. Although we started with an endovascular extraction, we converted to sternotomy under controlled circumstances due to the adhesion of the pacing leads to the surrounding tissue. Intraoperatively, surgeons excised the vegetations along with the attached chords and attempted a repair of the tricuspid valve. However, post-procedure echo showed severe TR with a flail anterior leaflet. We are unaware of guidelines or indications for open heart pacing lead extraction. The decision to replace a valve in a patient with ongoing sepsis is controversial and current recommendations are not suported by strong evidence. In most cases, decision is mainly left to the discretion of the attending physicians.