Study of the Anti-Anemic Activity of the Aqueous Extract of the Leaves of Petroselinum Crispum (Apiaceae) In the White Mouse (Mus Musculus) Of SWISS Strain
Petroselinum crispum (parsley) is a plant well known and much appreciated by African populations in general and Ivorians in particular. It is used for its anti-oxidant, antimicrobial, anti-diabetic, cardiovascular, anti-cancer and gastrointestinal properties.
The main objective of this study is to determine the antianemic activity of the aqueous extract of the leaves of Petroselinum crispum in anemic mice induced by phenylhydrazine.
Phytochemical analysis of Petroselinum crispum leaves revealed the presence of sterols, polyphenols, catechic tannins, alkaloids, saponosides and quinonic substances. On the other hand, gallic tannins and flavonoids were not detected in the aqueous extract.
Oral administration of aqueous extract of Petroselinum crispum at 200 mg / kg / day and 400 mg / kg / day to mice previously treated with phenylhydrazine increased the hemoglobin concentration, the number of red blood cells, the hematocrit level, indicating that the Petroselinum crispum leaves have anti-anemic activity.
These results partially support the traditional use of Petroselinum crispum in the treatment of anemia.
Keywords: Petroselinum crispum; Anemia, Phenylhydrazine
Anemia is a medical condition in which the number and size of red blood cells, or hemoglobin concentration, drops below a pla- teau level, affecting the ability of the blood to carry oxygen in the body [1]. It is the most frequently encountered public health prob- lem in the world, and particularly in developing countries [2]. The causes of this anemia are multiple among which, the deficiency of iron, certain vitamins such as vitamin B12, A and C as well as foliqueriboflavin acid that influence the formation of hemoglobin [3; 1]. Those most at risk for this disease are infants, children in intensive growth periods, elderly subjects, and pregnant women [4].
In Africa, this problem is not ignored, as on the continent, anemia is estimated to be responsible for 3.7% to 12.8% of maternal deaths during pregnancy and childbirth [4]. According to the study by Kokore and al. [5], the prevalence of the disease is 30.3% in Côte d’Ivoire, a sub-Saharan African country. Indeed, various studies provide indications, in children and women of childbearing age. The prevalence of anemia was estimated in 2012 to be 75% in children under 5 years of age, 54% in women of reproductive age, and 29% in men aged 15-49 years [6]. Face all these difficulties, researchers have increased their work on the therapeutic power of plants against anemia diseases.
Petroselinum crispum is a bright green annual herb, present in subtropical and tropical areas [7]. This plant is used for differ- ent treatments such as affections of the gastrointestinal tract, kidneys, lower urinary tract, and also to stimulate digestion [8]. Petroselinum crispum leaves are used for the treatment of amenorrhea, dysmenorrhea, kidney stones, prostatitis, diabetes, hyper- tension, hyperuricemia, constipation, odontalgia, pain, baldness and abortion induction in Spain [9]. They are also used as a food flavoring [10] as well as for the treatment of skin diseases [11] in China and Iraq, respectively. The aqueous extract of the leaves of Petroselinum crispum would have chemical constituents capable of treating anemia.
The equipment consists of biological materials, technical materials and chemicals.
The plant material consists of fresh leaves of Petroselinum crispum. The leaves were collected in August 2020 from the market of Adjamé in the city of Abidjan, Côte d’Ivoire
The animal species used is the white albino mouse Mus Musculus of SWISS strain aged four weeks of weight, ranging from 21 to 36 g. These animals were raised in the vivarium of the ENS. The animals were maintained and sexed in plastic cages with stainless steel covers. The cages were lined with wood shavings renewed every two days during the whole experiment. The animals were fed a standard rodent diet and received regular water.
Phenylhydrazine was used to induce anemia (60 mg/kg body weight);
- 0.9% sodium chloride (NaCl) was used for the various anemia induction doses
- Vitamin B12 was used as a reference substance.
The leaves of P. crispum were shade dried, and ground using an electric grinder, to obtain a coarse powder. The total aqueous extract (TAE) of P. crispum leaves was prepared following the method adopted by Yapo and al., [12]. 50 g of leaf powder was diluted in 1 liter of distilled water in the blender. The P. crispum powder added to one liter distilled water was homogenized with a blender three times for three minutes. The obtained homogenate was filtered once with a checkered cloth to remove the solid residues. The filtrate obtained was filtered five (5) times successively on hydrophilic cotton. The filtrate obtained was placed in an oven at 55°C for 48 hours to obtain a dry extract.
The phytochemical screening was carried out on the aqueous extract according to the methods used by N’guessan and al., [13]. It required various reagents. The research of catechic tannins was possible thanks to Stiasny’s reagent and sodium acetate. For the characterization of gall tannins, Stiasny’s reagent, sodium acetate and ferric chloride were used. Acetic anhydride and concentrated sulfuric acid were necessary for the investigation of sterols and polyterpenes. Hydrochloric alcohol diluted two (02) times, magnesium chips and isoamyl alcohol were used to search for flavonoids. The alcoholic solution of ferric chloride at 2% allowed the characterization of polyphenols. Bornstraëgen reagent, chloroform, 2-fold diluted ammonia and hydrochloric acid were used to search for quinone substances. Alkaloids were characterized using 60° alcohol, Burchard’s reagent (iodine-iodide reagent) and Dragendorff’s reagent (potassium iodo-bismuthate reagent). The presence of foams after shaking the aqueous solution of the extract allowed the identification of the saponosides.
Anemia was induced in mice by intraperitoneal administration of phenylhydrazine at 60mg/kg for 2 days (day 0 and day 2). Anemia was observed on day 3 by measuring hematological parameters that are red blood cell, hemoglobin and hematocrit. Mice that developed anemia with a hemoglobin concentration of less than 13 g/dl were enrolled in the study.
Blood samples were collected in EDTA tubes by puncture of the retroorbital plexus using capillary tubes twice on day 0 and day 15. The collected blood was analyzed for various hematological parameters only once.
White blood cells (WBC), red blood cells (RBC), hemoglobin concentration (Hb), blood platelets (PLT), and hematocrit (HCT) were analyzed using an automated analyzer. Red blood cells were used to assess anemia
The body mass of the mice was recorded during the experiment on day 0 and then every other (2) for two weeks.
Anemia was induced in all groups of mice except group 1 (negative healthy control), received an intraperitoneal injection of phenylhydrazine at 60mg/kg twice (days 0, 2) and treated the following day (day 3).
Mice were divided into five groups of six mice each, as follows:
Group 1: Control (healthy) served as a control and received distilled water.
Group 2: Untreated positive control receiving distilled water for two weeks.
J Blood Disord Ther
Group 3: treat with a reference molecule, vitamin B12 syrup at a rate of 1 ml/kg body weight (according to the dosage) diluted at 1/2
Group 4: Mice were treated with aqueous extract of P. crispum leaf at a dose of 200mg/kg P.C. daily for two weeks.
Group 5: were treated with the liquid extract of the leaves Petroselinum crispum at a dose of 400 mg/kg of bw per day for two weeks.
After oral administration of the different doses of aqueous extract of P. crispum, the mice were weighed, the animals were anesthe- tized with ether (Cooper) and then sacrificed. Blood samples were taken (day 15) for hematological analysis at the SAMU (CHU of Cocody-Abidjan). This analysis consisted in determining the effect of phenylhydrazine and aqueous extract on blood parameters (number of white blood cells, platelets and red blood cells, percentage of lymphocytes, neutrophils, monocytes, eosinophils and basophils, concentrations of Hb, PCV, MCV, MCH and MCHC)
The statistical processing of the results is done with the Graph Pad Uninst Prism 7 software. The results are given as mean ± followed by the standard error on the mean (mean ± SEM). Analysis of variance (ANOVA) followed by Tukey’s multiple comparison test, as well as the students’ T-test at the 5% threshold (P<0.05) to assess the significance of the differences observed. This software is also used to obtain the graphs.
The results of the phytochemical analysis showed the presence of sterols, polyphenols, gall tannins, quinonics, alkaloids and sapono- sides in the aqueous extract of Petroselinum crispum. However, catechic tannins and flavonoids were not detected in the extract.
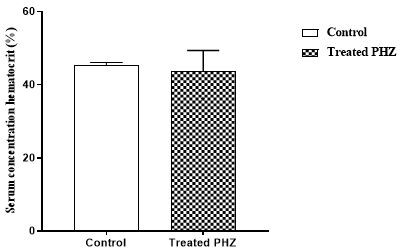
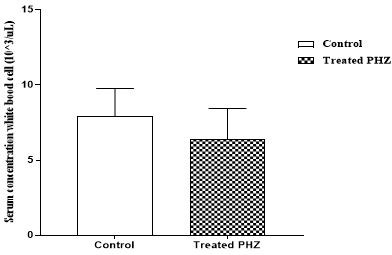
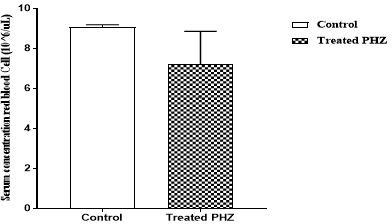
Phenylhydrazine caused a significant (p<0.05) decrease in red blood cell, hemoglobin, and hematocrit concentrations. Indeed, the red blood cell level decreased from 9.06 ± 0.07 10/μL3 to 7.20 ± 0.96103 /μL (graph 1). The hemoglobin level decreased from 13.47 g/dL± 0.09 to 9.48 ± 3.93 g/dL (graph 2). As for the hematocrit value, it was reduced from 45.47% ± 0.38 to 43.77% ± 3.27 (graph 3). However, administration of phenylhydrazine to the animals had no significant effect (p>0.05) on the white blood cell and platelet levels (graph 4 and 5).
The body mass of the animals in the healthy control group increased progressively throughout the treatment period. However, the body weight of the other groups (EAPcEAP200, 400 and vitamin B12) did not change significantly from day 0 to day 8. On the other hand, from day 10 onwards, the body weight of the 200 mg/kg bw group increased insignificantly (p<0.05) compared to the PHZ control. Moreover, it was only from day 12 onwards that a significant increase (p<0.01) was observed in the groups that received the aqueous extract at a dose of 400 mg/kg bw and vitamin B12 (graph 6).
The body mass of animals in the healthy control group increased progressively throughout the treatment period. In contrast, while the body mass of the animals receiving distilled water gradually decreased, the body mass of the animals in the groups receiving aqueous extract and vitamin B12 did not vary throughout the treatment period. (Graph 7).
After 14 days of treatment with the aqueous extract of petroselinum crispum leaves and vitamin B12 caused no significant change (p>0.05) in the white blood cell count compared with the healthy control and PHZ control (graph 8).
Treatment of anemic animals with EAPc and vitamin B12 restored red blood cell levels compared to those treated with distilled water. For the 200 mg/kg bw and 400 mg/kg bw doses the red blood cell count increased from 8.52 ± 0.13/μL.103 to 9.01 ± 0.13/ μL.103 and from 8.52 ± 0.13/μL.103 to 9.32 ± 0.12/μL.103, respectively. As for vitamin B12, the red blood cell level increased from 8.52 ± 0.13/μL.103 to 9.18 ± 0.22.10/μL3 (Graph 9).
Hemoglobin levels were significantly increased after treatment with aqueous extract at 400 mg/kg bow (p<0.01) and vitamin B12 (p<0.05) compared to PHZ controls. However, treatment of anemic animals with 200 mg/kg CP had no significant effect on hemo- globin levels compared to the PHZ control (Graph 10).
Mean hematocrit values were significantly (p<0.001) increased in anemic animals treated with aqueous extract at 400 mg/kg PC and vitamin B12 compared with those treated with distilled water. Indeed, aqueous extract and vitamin B12 promoted an increase from 48.7 ± 0.42.10/μL3 to 53.38 ± 0.59.10/μL3 and 48.7 ± 0.42 to 53.78 ± 0.80 in hematocrit, respectively (Graph 11).
There were no significant differences (p>0.05) in the number of blood platelets between control and test substance-treated batches (Graph 12).
After 14 days of treatment with aqueous extract of Petroselinum crispum leaves and vitamin B12 the relative weight of organs (kidney, heart, liver) vary little compared to healthy control and PHZ control. However this variation is not significant (p>0.05). On the other hand, the spleen weight of the animals increased insignificantly (p<0.05) (Table 1 and 2).
Phytochemical analysis revealed the presence of sterols, polyphenols, tannins, alkaloids and saponosides. However, the E.T.A of P. crispum does not contain catechic tannins and flavonoids. Moreover, the absence of catechic tannins and flavonoids observed during this study, to be explained by the method of phytochemical extraction used.
In their study on phytochemical screening, antibacterial and cytotoxic activities of P. crispum leaves, Al-Hadi and al., [14] also revealed, the presence of polyphenols, tannins, alkaloids and saponosides. It has been reported that saponosides and alkaloids possess anti-anemic potential [15]. Saponosides are also known to inhibit platelet aggregation and thrombosis. Plants containing saponosides have been successfully used in the management of liver inflammation, as tonics and sedatives, and to promote and revitalize blood circulation [16; 17]. The presence of these different chemical compounds in P. crispum E.T.A. would explain the common use of P. crispum in traditional medicine.
In this study phenylhydrazine was used at a rate of 60 mg/kg body weight to induce hemolytic anemia intraperitoneally in mice, it was found a significant decrease in the concentration of hemoglobin level, the concentration of hematocrit level and the number of red blood cells. These results corroborate with those of Tovi and al. [18], who observed after the administration of phenylhy- drazine, the reduction of hematological parameters. Phenylhydrazine is toxic to the organism, therefore causes health disorders, damages different tissues in the body and causes lysis of red blood cells [19; 20]. Phenylhydrazine has been reported to cause oxidative damage to red blood cells by increasing the formation of reactive oxygen species [21; 22].
The results of our work showed that the aqueous extract of P. crispum restored the hematological parameters in anemic mice after 14 days of treatment. Oral administration of aqueous extract of P. crispum at the dose of 200 mg/kg bw failed to regulate the anemia in the animals. However, the 400 mg/kg bw dose seemed to be better than the 200 mg/kg CP dose. The 400 mg/kg bw dose significantly increased the hemoglobin concentration, hematocrit concentration and red blood cell count compared to the PHZ control. A report indicates that oral administration of Tectona grandis (Lamiaceae) extract to rats, previously treated with phenylhydrazine, increased hemoglobin concentration, red blood cell count, hematocrit and reticulocyte count [23]. Veena and al., [24] also reported that the combination of aqueous extract of Azadirachta indica (Meliaceae) and Emblica officinalis (Eu- phorbiaceaea) produced significant anti-anemic activity. Indeed, the aqueous extract was administered for 15 days at a dose of 200 and 400 mg/kg body weight in rats previously treated with 60 mg/kg bw of PHZ. The results indicated a significant increase in hematological parameters. In addition, a study on the anti-anemic potential of three plant extracts namely Mangifera indicica (Anacardiaceae), Telfairia occidentalis (Cucurbitaceae), Amaranthus hybridus (Amaranthaceae) on phenylhydrazine induced anemia in rabbits showed an anti-anemic effect. Phytochemical analysis of these extracts detected saponins, tannins, cardiac glycosides, flavonoids and alkaloids, [25].
The differences in the anti-anemic potential of the plant extracts could be due to the different phytochemicals present: namely saponins, tannins, alkaloids and flavonoids which protect cells as powerful antioxidants and prevent or repair damage to red blood cells by free radicals or highly reactive oxygen species. Phytochemical screening of the extract revealed the presence of saponosides and alkaloids. Thus, it appears that the presence of these antioxidants in plant extracts reverses the harmful effect of phenylhydrazine.
Petroselinum crispum has been shown to be rich in iron and several minerals (Fe, Na, K, Ca, Cd, Cu, Cr, Mn, Zn) [26]. These results partially reflect the popular claim that parsley is an iron-rich herb. Iron serves as the core of the hemoglobin molecule, which is the oxygen-carrying component of red blood cells. The ability of red blood cells to carry oxygen is attributed to the pres- ence of iron in the hemoglobin molecule. The presence of iron is believed to be the source of the normal recovery of hemoglobin and red blood cells.
Regarding the weight of the mice, a reduction in body weight was observed in the PHZ control mice after 14 days of treatment with distilled water compared to the mice that received P. crispuim E.T.A. Decreased body weight is one of the indications of anemia, which is due to a lack of appetite in mice. Although P. crispum is rich in phytoconstituents such as sterols, alcohols, saponosides, gall tannin and polyphenols, it also contains a level of ion such as Fe, Na, K, Ca, Cd, Cu, Cr, Mn, Zn, the weight of animals treated with P. crispum E.T.A. varies little compared to PHZ controls [26].
Our study showed that the spleen weight of animals previously treated with PHZ increased in volume except for the healthy control group. Our results are consistent with those of Pham-Quang Chi, [27] who reported that phenylhydrazine increased the weight and volume of the spleen and some organs such as kidney, liver and adrenal glands. Ferrali and al., [28] reported that phenylhydrazine, by increasing the concentration of hemolysis, leads to an increase in erythropoietin, ferritin and ferrous levels. Hemolysis causes hypertrophy of the spleen and chronic failure due to hypertrophy of the spleen cells.
Injection of PHZ to mice produced hemolytic anemia characterized by reduction of hematological parameters especially hemo- globin concentration, hematocrit concentration. This study indicated that the aqueous extract of petroselinum crispum leaves increased, after 14 days treatment, the rate of hematological parameters significantly with 400 mg/kg bw/day.
This study provides scientific evidence and credibility to the effectiveness of Petroselinum crispum extract in the management of anemia.

.JPG)