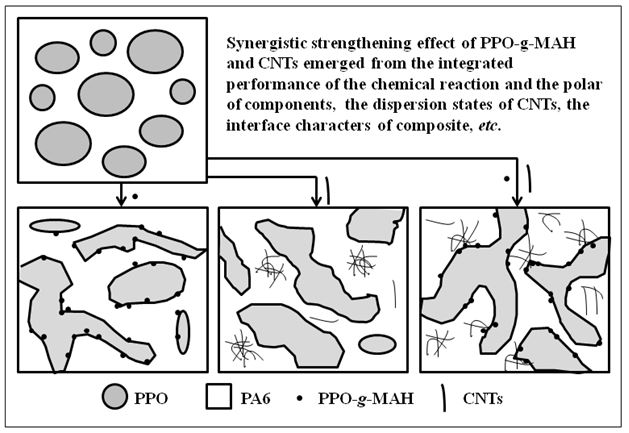
Synergistic Strengthening Effect of Poly (2,6-Dimethyl-1,4-Phenylene Oxide) Grafted with Maleic Anhydride and Carbon Nanotube on PPO/PA6 Blend
PPO/PA6/CNTs composites were prepared through melt blending. The morphology, mechanical properties and rheological behaviors of PPO/PA6/CNTs composites were researched. In order to increase the mechanical properties of PPO/PA6/CNTs composites, PPO-g-MAH, as the compatibilizer, was introduced. The compatilization of PPO-g-MAH and the selective dispersion of CNTs made a phase transformation from “sea-islands” to co-continuous. The interfacial bonding between PPO and PA6 was improved obviously, because of the reaction between the anhydride groups of PPO-g-MAH and the amino-terminated groups or carboxyl-terminated groups of PA6. And the mechanical properties of PPO/PA6/CNTs composites improved after adding PPO-g-MAH, especially for the impact strength. The synergistic strengthening effects of PPO-g-MAH and CNTs increased the storage modulus, loss modulus and viscosity of PPO/PA6 blend in dynamic rheological test, reflecting the compatibilized effect of PPO-g-MAH and the confined effect of CNTs.
Keywords:Strengthening; Synergism; Compatibility; Carbon nanotube (CNT)
As a polymer alloy with high performance, PPO/PA alloys are applied extensively in electronic-electrical areas, automotive industry, office equipment, etc. The researches about strengthening PPO/PA alloys have been regarded for a long time. It is a potential method to achieve PPO/PA blends with high mechanical strength through introducing nanoparticles, such as LCP, POSS, GO, C60 and so on [1-5].
In our previous work, it was found that the addition of carbon nanotubes (CNTs) led to the evolution of phase structure from "sea-island" to co-continuous in PPO/PA6 blend [6]. But the mechanical strengh did not improve as much as we thought, because the bad dispersion state of CNTs in matrix and the weak adhensive force between CNTs and polymers.
Adding a reactive compatibilizer is often helpful to improve the morphology and interface properties [7-9]. These compatibilizers are block or graft copolymer, with two characteristics in their molecular structures: one end has the same or similar structure as PPO (or PS); the other end has the reactive groups with the amino-terminated groups and carboxyl-terminated groups of PA (for example, elastomers, acid anhydride and epoxy) [6,10-14]. In this work, poly (2,6-dimethyl-1,4-phenylene oxide) grafted with maleic anhydride (PPO-g-MAH) was added in PPO/PA6/CNTs composite to improve the interface properties, and then increase the strengthening effect of CNTs in PPO/PA6 blends.
Poly(2,6-dimethyl-1,4-phenylene oxide) (PPO, code Noryl 731) was purchased from Sabic GE Plastics Co.USA, with the Mw of 605,000 g/mol (determined by gel permeation chromatography, GPC), the melt flow rate (MFR) of 9.2 g/10min (tested at 280 °C with the load of 5.0 kg, according to ASTM D1238) and the density of 1.06 g/cm3 (according to ASTM D792). Polyamide 6 (PA6, code CM1017) was produced by Toyolac, Japan, with the Mw of 627,000 g/mol (determined by GPC) and the density of 1.12-1.14 g/cm3 (according to ASTM D792). PPO-g-MAH (GPm 450D) was obtained from Ningbo Nengzhiguang Co. China, with the grafting ratio of 1.0-1.5 wt%. CNTs, with an average diameter of 20±5 nm and a length of 1-10 μm, were prepared by catalytic chemical vapor deposition (CCVD) method and provided by Chengdu Organic Chemicals Co. (Chengdu, China).
PPO and PA6 were dried at 85 °C in a vacuum oven for 24 h, and then PPO/PA6/PPO-g-MAH/CNTs composites were melt blending using a Thermohaake rheomixer (Haake Torque Rheometer, model Polydrive, Germany) at 240 °C for 10 min with a rotor speed of 70 rpm (Table 1). The blending composites were transferred to compression mold at 240 °C for 15 min with the press of 15 MPa and then cold pressed for 30 min with the press of 15 MPa to obtain samples for testing.
Morphology of different CNTs-x was achieved through JEM-1230EX transmission electron microscopy (TEM, JEOL, Japan) after sliced to 50-100 nm. Morphology of PPO/PA6/blends was observed through S-570 scanning electron microscopy (SEM, Hitachi, Japan) under an accelerating voltage of 3 kV, and the disperse state of CNTs in matrix was analyzed through S-4800 SEM (Hitachi, Japan) under an accelerating voltage of 5 kV.
Impact tests were carried out by a XCJ-4 impact instrument according to GB1043-93(similar to ISO179-1993), with the specimens were 70×6×4 mm3 (with a notch of 3.0 mm in width and 2.0 mm in depth). Tensile tests were carried out on an Instron tensile tester (Model WDW-10) according to GB1040-89 (similar to ISO527-1993), with the crosshead speed was 50 mm/min and the gauge length was 50 mm. All the tests were performed at 23±3 °C. Five measurements on a sample were averaged.
Rheological measurements were preceded by an Advanced Rheology Expansion System (ARES). An isothermal dynamic frequency sweep was conducted with a disk of 1.2mm thickness and 25mm diameter at a frequency range of 10-2 to 102 rad/s, strain amplitude of 1% and a temperature of 240 °C.
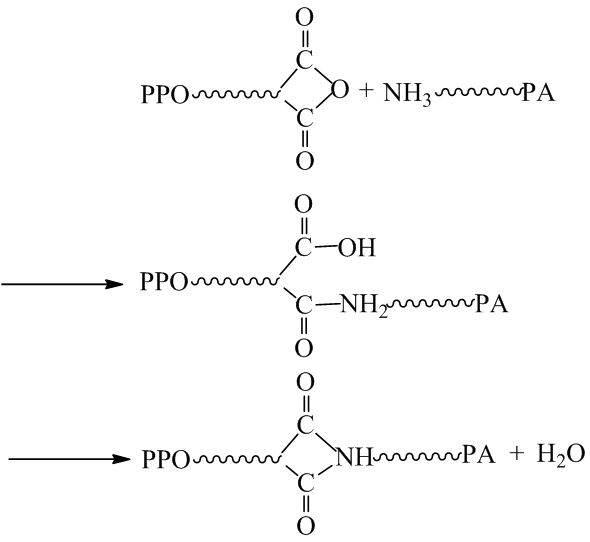
For PPO/PA6 blend, a typical matrix-droplet structure was achieved as in Figure 1A. PPO acted as dispersed phase and PA6 as continuous phase, because PPO had higher melt viscosity than PA6. When PPO-g-MAH was added in PPO/PA6 blend, a co-continuous phase structure was observed (Figure 1B). The amino groups of PA6 attacked the anhydride groups of PPO-g-MAH to form a graft copolymer, i.e. PPO-g-PA6, which increased the viscosity of PA6 (Scheme 1). When the viscosity of PA6 increased to approach the viscosity of PPO, the PPO phase converted into the continuous phase. The PPO-g-MAH acted like a surfactant to reduce the interfacial tension and the interface activity, which also promoted the phase transition from “sea-islands” to co-continuous.
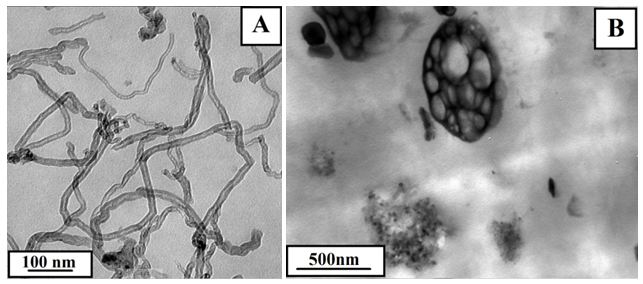
The surface of CNTs was smooth as shown in Figure 2A and the dispersive state of CNTs in PPO/PA6 was appeared in Figure 2B. Because CNTs dispersed selectively in PA6 phases due to the lower viscosity of PA6 compared to PPO, which increased the viscosity of PA6 (Figure 2B). When the viscosity of continuous phase (PA6) was the nearly same as the dispersed phase (PPO), the movements of PPO chains became easier and PPO phase began the transition from dispersed phase to continuous phase.
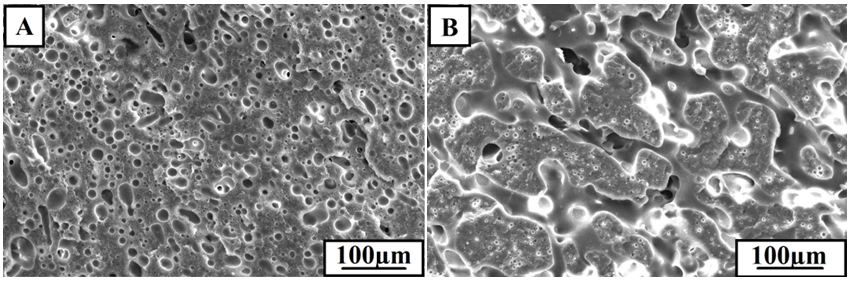
For PPO/PA6 blend, a typical matrix-droplet structure was being achieved. PPO acted as dispersed phase and PA6 as continuous phase, because PPO had higher melt viscosity than PA6 (Figure 3A). The interfaces between PPO and PA6 were distinct, and the dimensions of most droplets were over 5 μm. However, when PPO-g-MAH was added, as shown in Figure 3B, the phase interfaces between PPO and PA6 became obscure and the sizes of dispersed phases decreased obviously. And when CNTs were added, the co-continuous phase structure was observed clearly and the matrix-droplet morphologies were no longer apparent (Figure 3C and D). CNTs dispersed selectively in PA6 phase due to its lower viscosity, which increased the viscosity of PA6. When the viscosity of continuous phase (PA6) was the nearly same as the dispersed phase (PPO), the movements of PPO chains became easier and PPO phases began the transition from dispersed phases to continuous phases. Comparing with PPO/PA6/CNTs composite (Figure 3). The impact fracture surface of PPO/PA6/PPO-g-MAH/CNTs composite was no longer so clear, but rather somewhat stratified, appearing a better ductile fracture (Figure 3D).
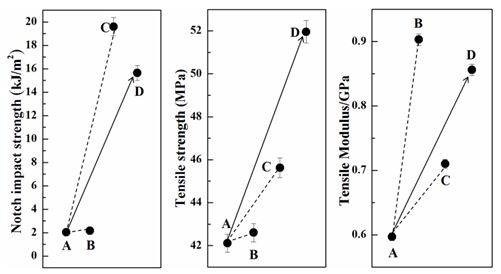
Only adding CNTs in PPO/PA6 blend Figure 4B, the tensile strength and tensile modulus increased comparing with PPO/PA6 blend Figure 4A, due to the strengthening mechanism of CNTs. And the impact strength had no obvious changes due to the weak interaction between CNTs and matrix as shown in Figure 3C, in which many single tubes were drown out.
The compatibilized effect of PPO-g-MAH was in favor of increasing the interaction between PPO and PA6 phases, improving the tensile modulus, tensile strength and impact strength obviously of PPO/PA6/PPO-g-MAH (Figure 4C). Especially impact strength increased from 2.0 to 19.62 kJ/m2. PPO-g-MAH served as an effective compatibilizer to decrease the interfacial tension and increase the interface adhesion, so the phase size decreased in blends and two phases mixed evenly (Figure 3B).
However, when PPO-g-MAH and CNTs were both added in PPO/PA6 blend, the impact strength, tensile strength and tensile modulus all increased obviously, especially for the tensile strength (Figure 4D). The tensile strength of PPO/PA6/CNTs was 42.6 MPa, the tensile strength of PPO/PA6/PPO-g-MAH was 45.2 MPa, and the tensile strength of PPO/PA6/PPO-g-MAH/CNTs was 52.96 MPa.
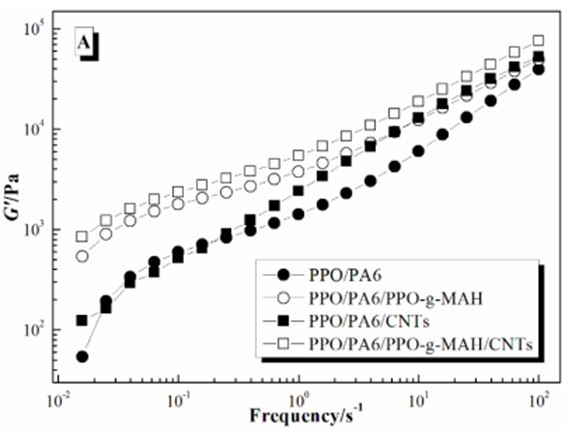
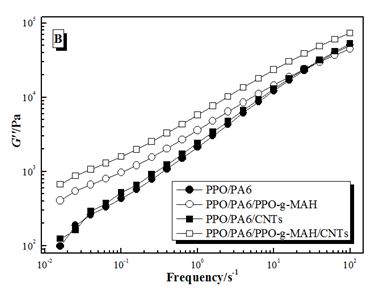
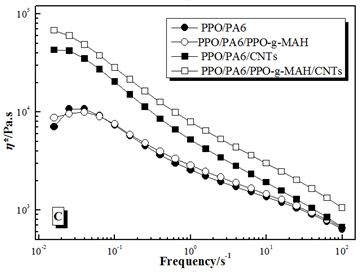
The storage modulus (G’), loss modulus (G’’) and complex viscosity (η*) of PPO/PA6, PP/PA6/ PPO-g-MAH, PPO/PA6/CNTs and PPO/PA6/PPO-g-MAH/CNTs composites in dynamic frequency sweeping was shown in Figure 5. The modulus (G’ and G’’) increased and the viscosity (η*) had little change after adding PPO-g-MAH in PPO/PA6 blends, especially at lower frequency. While the modulus had little change and the viscosity increased after adding CNTs in PPO/PA6 blends. When PPO-g-MAH and CNTs were both added, the modulus and viscosity both increased obviously.
For PPO/PA6/PPO-g-MAH blend, some PA6 chains concentrated at the interfaces by the reaction between PPO-g-MAH and PA6. And the effective movement and viscosity of those PA6 chains was restricted, resulting in the increase of modulus (Figure 5A and B). But the bulk viscosity of PA6 phases did not change, so the viscosity of PPO/PA6/PPO-g-MAH blend increased very little (Figure 5C).
For PPO/PA6/CNTs composite, the viscosity of PA6 phases remarkably increased because of the selective dispersion of CNTs, resulting in the increased of η* (Figure 5C). While the bad adhesive strength between polymers and CNTs made CNTs had little effects on the effective movement of macromolecules, so the G’ and G’’ had no significant changes (Figure 5A and B).
When PPO-g-MAH and CNTs were both added, PPO-g-MAH had a strong confinement effect to PA6 chains and CNTs, due to the reactions between PPO-g-MAH and PA6 and the attraction of polar anhydride groups for CNTs. Therefore, CNTs dispersed selectively in PA6 phases approaching interfaces (Figure 6). As a result, the viscosity of PA6 phases increased and the effective movement of macromolecules weakened, which caused the enhancement of G’, G’’ and η* (Figure 5C).
PPO-g-MAH or/and CNTs were introduced in PPO/PA6 blends via melt blending. The reaction between PPO-g-MAH and PA6 transformed the morphology of PPO/PA6 blend from "sea-islands" to co-continuous. And PPO-g-MAH, as the compatibilizer, improved the interfacial performance between PA6 and PPO. The co-continuous morphology and strong interface adhension improved the mechanical properties of PPO/PA6 blend and increased the confinement for polymer chains. CNTs also changed the PPO phases from dispersed phases to continuous phases. The selective dispersion increased the viscosity of PA6 phases and the bad interface performance made the limited improvement of mechanical properties. The synergistic strengthening effect of PPO-g-MAH and CNTs was reflected in mechanical properties and dynamic rheological behaviors, as the integrated results of the interface characters of composites, the dispersive states of CNTs, the interaction of components, etc.
This work was supported by the Ningbo Science and Technology Innovation Team (No. 2015B11005) and the Ningbo Natural Science Foundation of China (No. 2018A610102).

.JPG)
.JPG)