Synthesis and Characteristics of Metallic Nanoparticles Coated with Olive Oil
The original approach has been assisted for green synthesis and characterization of silver nanoparticles coated by extra virgin olive oil for improved functioning in medicine. Silver nanoparticles were synthesized using Aloe Vera extract. Synthesized silver nanoparticles were then coated by extra virgin olive oil for being the stabilizer and also for having improved antimicrobial activity. These silver nanoparticles were studied using different characterization techniques including UV Visible spectroscopy, X-Ray Diffraction (XRD) and Scanning Electron Microscopy (SEM). SEM showed that average size of synthesized silver nanoparticles is 55.73nm.
Keywords:AgNPs; Antimicrobial Activity; Olive Oil
Nanotechnology has opened a broad range of biomedical applications by adopting green chemistry for the synthesis of nanoparticles [1]. Use of green synthesis method (involving plants, microorganisms and templates) [2] is preferred on chemical and physical methods (chemical methods are unsafe for environment [3] and physical methods are expensive) [4]. This technique is most stable and one-pot process [5]. The nanoparticles synthesized by green synthesis are non-toxic [6].
Silver (in bulk form) can absorb oxygen in an oxygen-rich environment such as blood. This makes silver poisonous for bacteria which need oxygen for survival and thus silver compounds are being as antibacterial drug [7]. Silver nanoparticles (size range from 1nm to 100nm) [8] have surface/volume ratio much greater than the bulk material. Mode of existence and interaction with the bacteria surfaces is improved which results in higher antimicrobial activity [9]. The antibacterial properties of silver nanoparticles are being used for food storage, in medical devices, in home appliances for water treatment, in cotton fabrics and in many environmental devices [3].
Silver nanoparticles can be synthesized nanoparticles by using extract of aloevera (effective specie for medical purposes) [10] leaves. It is very important to control the size and shape of these synthesized silver nanoparticles. Stable collision suspension is usually challenging due to Vander Wall forces. Hence, it is better to coat them with a surfactant for better dispersion of nanoparticles. Oleic acid is a well-known surfactant for this purpose [11]. Some other carboxylic acids such as erucic acid and linoleic acid are also being helpful in this regard. Olive oil, being a good natural source for this surfactant is found to stabilize the nanoparticles. Table 1 shows the ratio of these acids present in olive oil [11].
Olive oil being used is obtained without any chemical reaction. This extra virgin olive oil can reverse the oxidative damage of brain and can control the aging problems. It minimizes the possibility of stroke, sudden cardiac death and myocardial infarction.It normalizes the anti-oxidant enzymes and hence minimizes the cancer risk. Cholesterol level is controlled by anti-oxidant and anti-microbial activity of the oil [4]. Hence silver nanoparticles coated by this olive oil can have better antimicrobial activity.
The whole experiment consisted of two main tasks. First task was to synthesize the silver nanoparticles while second task was to coat the synthesized particles by extra virgin olive oil.
10 g aloevera leaves were washed by distilled water and cut carefully. These leaves were crushed in marble mortar and pestle for 30 minutes and a paste was obtained. The paste was then added in 250 ml Erlenmeyer flask containing 100 mL distilled water. This mixture was then put in water bath for 15 minutes at 60. Extract was filtered with Whatman filter paper no 1 and was stored at -15 °C [8].
0.17grams of silver nitrate were added in 1000mL of distilled water in a 1500mL flask and 1m-M solution of AgNo3 was prepared as it was mixed by stirring rod it for 15 minutes. 150mL of 1 m-M solution of 50mL aloe Vera extract (extract was found to be higher than the plant) [12] was added by the help of a 5mL pipette with continuous stirring. A brown-yellow solution indicated the formation of silver nanoparticles.
Mixture was transferred in falcons which were put in laboratory centrifuge and it was centrifuged for 30 minutes at 2500rpm (resolution per minute). The particles were washed by distilled water for five times.
The confirmation of existence, size and shape of silver nanoparticles was done by using XRD, UV/VIS and SEM. UV spectra analysis was done by UV spectrophotometer 2800 present in Biotechnology Department GCU Lahore. The UV visible spectroscopy was carried out with resolution 300-700 nm for a 1mg/mL solution of prepared solution. X-ray Diffraction was done by Physics Department Comsats Lahore using PANalytical X’Pert Powder Diffractometer. X-rays of Copper K Alpha with wavelength 1.5406Å were used to study the structure and size of the synthesized silver nanoparticles. The surface morphology analysis of nanoparticles is informative for the size of nanoparticles. Size of silver nanoparticles was studied using Scanning Electron Microscopy. SEM was done by Comsats University Lahore using Tescan Vega 3 LMU for silver nanoparticles without coating while SEM for coated silver nanoparticles was done by Centre of Advanced Physics Studies CASP, GCU Lahore.
The very first evidence of the synthesis of silver nanoparticles through aloevera leaves were the changed color of the solution after 24 hours as shown in the Figure 1 .
Solution of silver nitrate changed its color in dark brown on addition of leaves extract while the color of the other solution remained unchanged.
The silver nanoparticles obtained from the solution were coated with extra virgin olive oil which were then water bathed by using distilled water.
Figure 2a shows solution before centrifugation while Figure 2b shows solution after centrifugation. In Figure 2b silver nanoparticles can be observed at the bottom of the falcons. Centrifugation was done after each bath. Water was removed carefully by the falcons and new distilled water was added in the falcon. 5mL water was added each time for water bathing of the coated silver nanoparticles
UV spectra analysis was done by UV spectrophotometer 2800 present in Biotechnology Department GCU Lahore.
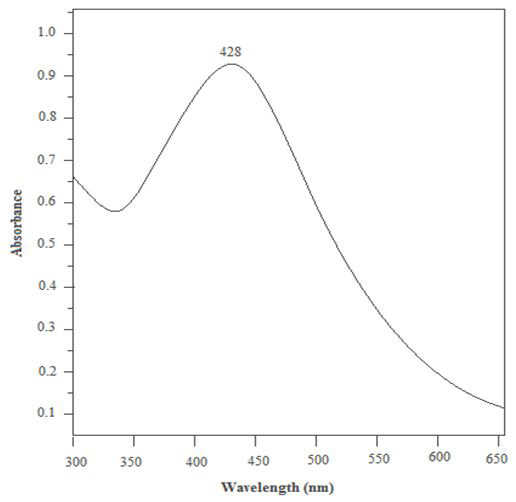
The UV visible spectroscopy was carried out for a 1mg/mL solution of prepared solution. A graph was plotted between wavelength range of 300-700 nm and absorbance. A strong peak was observed at 428 nm. This confirms that the peak is due to silver nanoparticles. This results matches with the existing literature according to which peak in the range of 410-450 nm proves the presence of spherical nanoparticles [13,14] (Figure 3 ).
Energy of visible and ultraviolet radiations is absorbed by the electrons of atoms and molecules. This energy excites the electrons and they go from a lower energy level to a higher energy level. Due to quantization of energy levels of matter, these transitions are possible only when a wave of specific energy interacts. Absorbance in the UV-visible spectroscopy graph is simply the amount of light absorbed by the solution.
X-ray Diffraction was done by Physics Department Comsats Lahore using PANalytical X’Pert Powder Diffractometer. X-rays of Copper K Alpha with wavelength 1.5406Å were used to study the structure and size of the silver nanoparticles synthesized by alovera leaves extract. Ni was used as filter and X-rays of Copper K Alpha were used to study the properties of silver nanoparticles at 30 keV/30 mA.
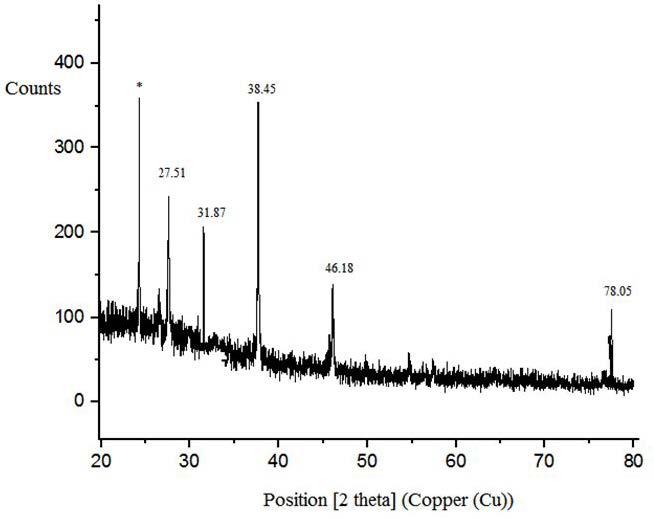
Obtained XRD pattern of the synthesized silver nanoparticles is shown in Figure 4 .
The data of the XRD graph is drawn using software Origin 6.0. Six peaks appeared in the XRD analysis graph. First peak appeared at 24.22° which represents nanoparticles of Zinc. Second peak appeared at 27.5°, third peak appeared at 31.87°, forth appeared at 38.45°, fifth appeared at 46.18° and sixth peak appeared at 78.05°, all represent existence of silver nanoparticles in the sample.
The diffraction peaks appeared at 27.51°, 31.87°, 38.45°, 46.18° and 78.05° refers to silver nanoparticles with planes (210), (113), (124) and (311) respectively and match with the data of JCPDS card number 04-0783. These hkl values are representing face-centered-cubic (FCC) structure of silver nanoparticles.
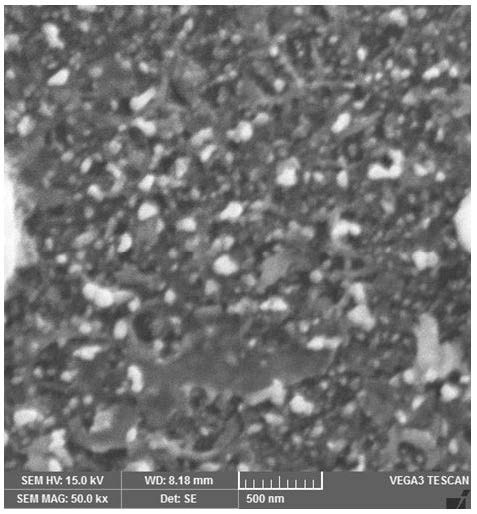
The surface morphology analysis of nanoparticles is informative for the size of nanoparticles. Size of silver nanoparticles was studied using Scanning Electron Microscopy. SEM was done by Comsats University Lahore using Tescan Vega 3 LMU for silver nanoparticles without coating while SEM for coated silver nanoparticles was done by Centre of Advanced Physics Studies CASP, GCU Lahore (Figure 5 ).
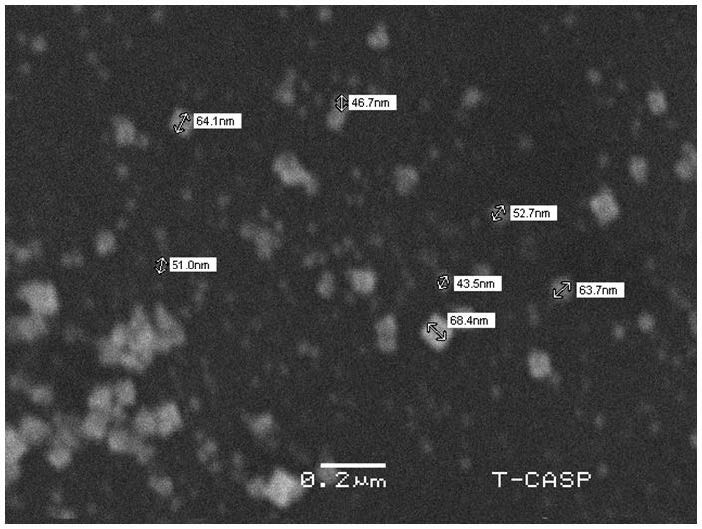
The range of the size of synthesized silver nanoparticles coated by aloevera is 43.5 nm to 68.4 nm. The morphology confirms the size of the synthesized particles in nm range. The average size of the particles is 55.73 nm. The homogeneity of the material that fabricated is indicated by the non-uniform surface behavior in the SEM sample (Figure 6 ).
Coating of olive oil acted as a stabilizing agent for green synthesized silver nanoparticles. Due to this, silver nanoparticles coated by olive oil were found to be less aggregated.
Small points in the picture shown above are coated silver nanoparticles while white crystal like structures is olive oil which remained uncoated.
Silver nanoparticles are successfully synthesized by aloevera extract. Nanoparticles without coating start aggregating and hence homogeneity is disturbed. Stabilizing agent which is being used for silver nanoparticles is PVP but it adds toxicity in silver nanoparticles. Extra virgin olive oil itself is an antimicrobial agent and coating of olive oil stabilizes silver nanoparticles. Hence these nanoparticles can be considered safer and more useful for Food Storage, Water treatment, Environmental devices, Bone cements, Biosensors, Medical devices and Optical data storage.