The Association between the Methyltransferase DNMT3A and Cancer
Cancer initiation and progression is controlled by both genetic and epigenetic events; DNA methylation alteration is one of the critical events for malignant cellular transformation. Aberrant in DNA methyltransferases (DNMTs) mainly mutations in the gene encoding DNA methyltransferase DNMT3A were reported in patients with cancer. Mutations in the DNMT3A have been demonstrated to lead to lung tumors, colorectal cancer, breast cancer, ovarian cancer, esophageal squamous cell carcinoma, hepatocellular carcinoma, and pancreatic cancer. Here, we provide an overview of two recently discovered DNMTs, DNMT3A, and DNMT3B, that is necessary for de novo methylation. Consequently, DNMTs have potential utility as anti-cancer targets. Here, we summarize past and recent insights regarding DNMTs and we share the current knowledge about DNMT3A and DNMT3B and their possible implications in treating cancer. We also review the clinical findings regarding the importance of DNMT3A in different types of cancer over the past years and present a roadmap for further research designed to develop strategies for identifying and implementing novel therapeutic targets.
Keywords:DNMT3A; DNA Methylation; Ovarian; Lung; Carcinoma; Leukemia
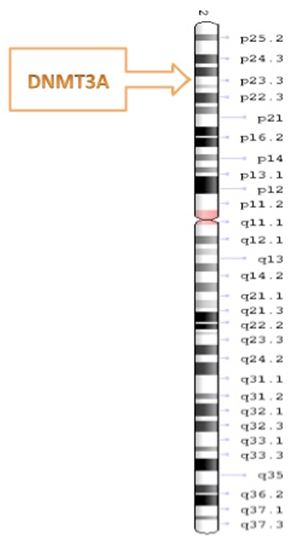
Cancer defined as a malfunction or disordered process of cell division and is a disease type that appears to strike without warning. It involves changes in the structure of DNA that promote alterations of normal DNA regulatory mechanisms [1]. Abnormalities in DNA methyl transferases (DNMTs)—namely DNMT1, DNMT3A, and DNMT3B—have been observed in many different types of malignancies [2]. DNMT3A is a 130-kDa protein that is located on the 2p23 chromosome in humans and is coded by 23 exons [3]; it consists of an amino-terminal domain that is unique to the long isoform and exhibits a DNA-binding capability [4-6]. DNMT3A mutations have been found to be associated with elevated numbers of platelets and bone-marrow blasts [6]. Mammalian DNMTs play a role in determining methylation patterns throughout gametogenesis, embryogenesis, and substantial tissue advancement [7]. The DNMTs family are classified into three sub-categorizes, namely DNMT1, DNMT2, and DNMT3 with catalytic activity [8]. DNMT1 has been found to be the most abundant member of the DNMT family and is involved in the maintenance of methylation [9]. DNMT2 is considered to be RNA methyl transferases specifically tRNA, however the role of DNMT2 in DNA methylation has been controversial [10,11]. Whereas DNMT3 functions as a de novo methyltransferase and includes two associated proteins encoded by distinct genes, namely DNMT3A and DNMT3B [10]. The related DNMT3-like (DNMT3L) protein functions as an extra protein component in conjunction with DNMT3A in the processes of embryonic development and genomic imprinting but lacks a catalytic domain [12,13]. A mutation in DNMT3A related to malignancy was first identified in 2010 [14,15]. DNMT3A and DNMT3B have been shown to be necessary for de novo methylation and for mouse development. Correspondingly, deactivation of both of these genes via gene targeting has been found to prevent de novo methylation in embryonic stem cells and early embryos [16]. The DNMT3 enzyme plays crucial roles in maintaining the DNA methylation pattern found at the replication-fork site and in the methylation of newly biosynthesized DNA [17]. The most widely observed type of missense mutation in DNMT3A affects the amino acid at residue R882 and accounts for 60% of DNMT3A mutations [18-20]. Although the DNMT3A gene normally plays a role in preventing malignancy, mutations in this gene have been identified as playing a role in the development of hematological neoplasms [21].
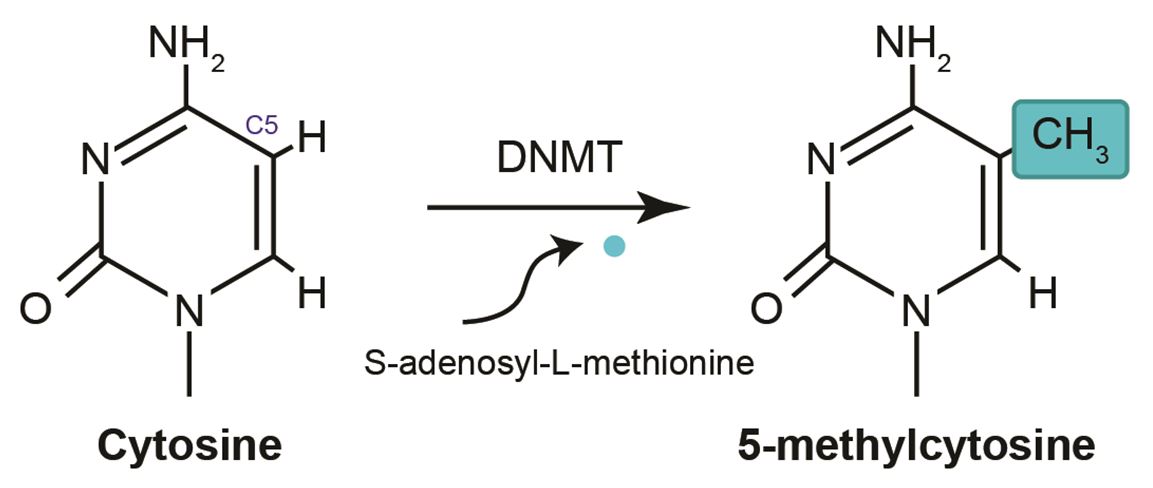
DNA methylation is an epigenetic alteration that is critical in development, imprinting, stem-cell management, and X-chromosome inactivation [22]. Epigenetic pathways can monitor gene expression by adjusting both the spatial extent of DNA methylation and modification (e.g., acetylation, methylation, and phosphorylation) of histone residues of surrounding nucleosomes in which the DNA double helix becomes twisted [8]. Epigenetic alterations can be one of the roles to begin in a disease [23]. Methylation of DNA entails the addition of a methyl (CH3) group to the C5 portion of the pyrimidine ring of cytosines to generate 5-methylcytosine (5mC; Figure 1) [24]. In the context of CpG-dinucleotide, pair’s aberrant DNA methylation is one of the most well-known epigenetic events associated with human cancers [17]. High DNA methylation related to the silencing of gene expression [25]; however, the mechanisms underlying the development of methylation abnormalities and their pathological importance are still not well-understood [21]. The methyl cytosine-dioxygenase proteins TET1, TET2, and TET3, convert the 5mC to 5-hydroxymethylcytosine (5hmC) [26]. 5hmC should be retained by DNMT1 if not, it will leads to passive demethylation through cell division, and thus provides a mechanism for DNA methylation [21,27,28]. DNA methylation also plays a role in other essential processes that are involved in genomic imprinting, suppression of retrotransposon elements, and X-chromosome inactivation, which are all important for normal development [16,29].
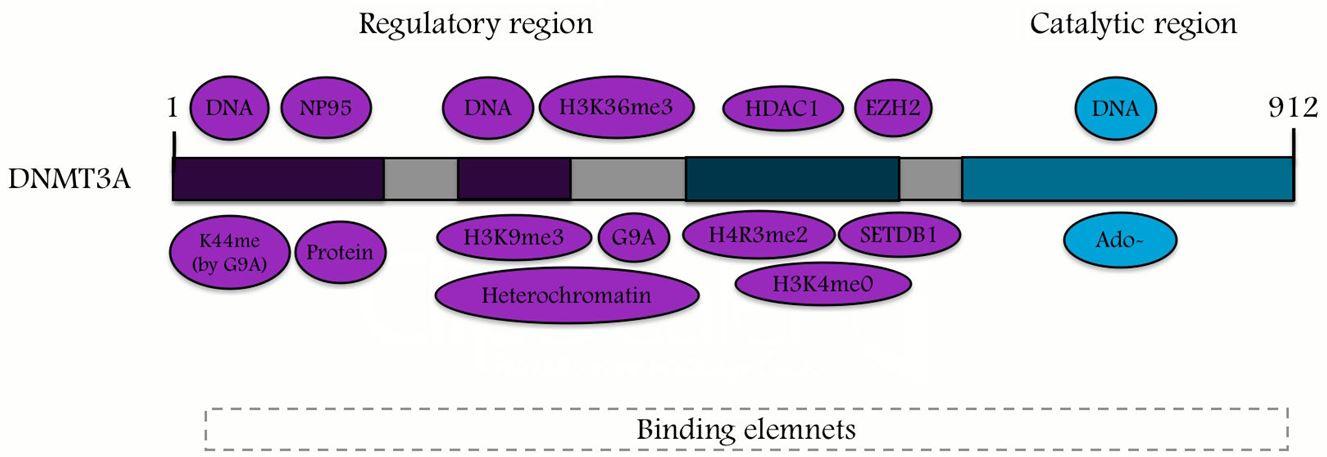
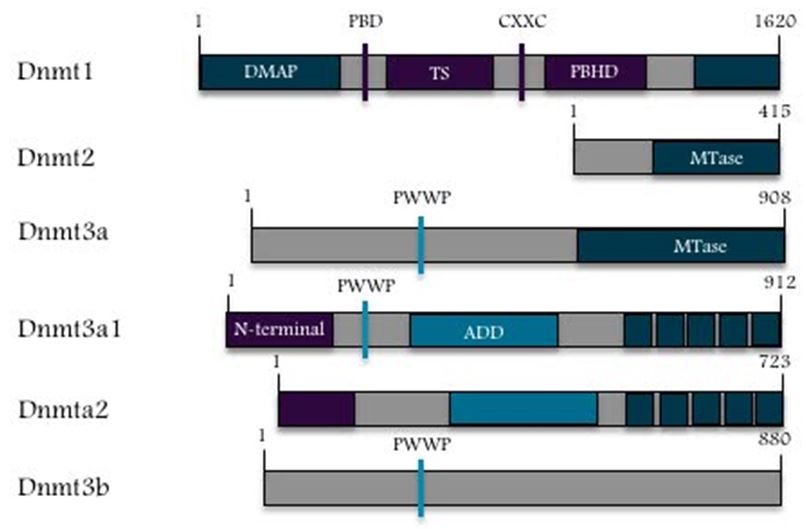
DNMT3A is located on chromosome 2 at p23 in humans comprised of 23 exons, the DNMT3A gene is translated into a 130-kDa protein that is expressed in different tissues and cells [3]. Figure 2 shows the Genomic determination of the DNMT3A gene of human. DNMT3A contains 3 main structure domains: a proline-tryptophan-tryptophan-proline (PWWP) domain, an ATRX, DNMT3, and DNMT3L-type zinc finger (ADD) domain, and the methyltransferase (Mtase) [30]. DNMT3A is a highly conserved protein in mammals, as it exhibits a 98% homology between human and murine homologs [31]. The protein molecule of DNMT3A2 contains the following major domains: the ATRX-DNMT3-DNMT3L (ADD) domain, Pro-Trp-Trp-Pro (PWWP) domain, and catalytic methyltransferase domain [21]. During transcriptional repression, there is an interaction between the ADD and PWWP domains and proteins [21]. Thus, the N terminus might be involved in DNA binding [4,32]. The DNMT3A catalytic domain is inhibited by the ADD domain. This process can occur via the formation of an auto-inhibitory loop that is released because of interaction with the unmodified lysine 4 of histone H3 (H3K4me0) [21], thereby connecting DNMT3A and H3 chromatin marks [33]. Figure 3 shows that DNMT3A contains an amino terminal domain that is considered to be unique to the long isoform, which consist of two regions the catalytic and regulatory region [4,5]. Figure 4 shows the two major fragments of the murine RNA isoforms, in which the protein lengths are shown in terms of the numbers of amino acids; the long fragment is Dnmt3a1 and the short fragment is Dnmt3a2 [21]. In DNMT3A2, the first six exons of the amino-terminal domain are missing, and its expression is limited to embryonic stem cells, testes, ovaries, spleen, and thymus [34,35].
DNMT3A plays an important biological role in self-renewing cells, allowing their differentiation [36]. DNA methylation is established by DNMTs, which catalyze the addition of the methyl group to the C5 (carbon 5) position of cytosine to generate a C5-methyl-cytosine (5mC) (see Figure 1 above). Until recently, only two types of DNMTs had been identified, namely de novo methyltransferases and maintenance methyltransferases. De novo methyltransferases catalyze the formation of hemi-methylated CpG dinucleotide sites in double-stranded DNA and are responsible for maintaining the methylation pattern. Maintenance methyltransferases play a role in the addition of a methyl group to DNA when one strand has previously been methylated and are responsible for maintaining the methylation patterns that have been generated by the de novo methyltransferases. Commensurate with its essential function, the patterns of DNA methylation are well controlled and show tissue specificity. Illingworth, et al. and Okano, et al. have shown that two recently discovered DNMTs, namely DNMT3A and DNMT3B, are necessary for de novo methylation and for mouse development [16,37]. Deactivation of both genes via gene targeting prevents de novo methylation in embryonic stem cells and early embryos.
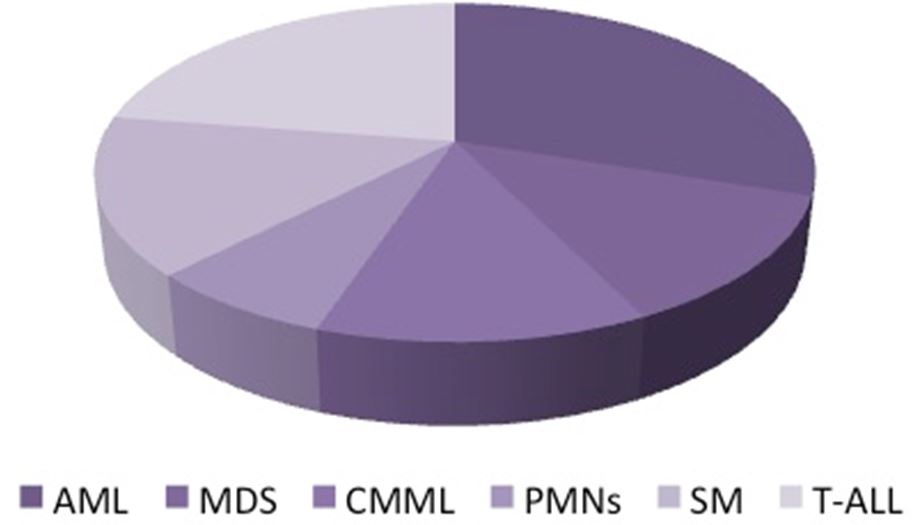
Biological processes typically function normally until perturbed by abnormalities such as those characterizing hematological malignancies (e.g., leukemia). These changes will alter the organization of reproduction, proliferation, recognition, and survival of human hematopoietic stem cells even though the hematopoietic microenvironment is not severely damaged [8]. Hematologic malignancies can be significantly related to gene mutations as it widely studied in Cancer Genome projects [38,39]. There are different types of hematologic malignancies, including lymphoma, leukemia, myelodysplastic syndrome, and myeloproliferative diseases, which result in uncontrolled clonal expansion of hematopoietic stem/progenitor cells [8]. Patients with acute myeloid leukemia (AML) are characterized by the occurrence of two major classes of DNMT3A mutations, both of which are associated with a repeated set of mutations at R882 domain [18,38]. A mutation of R882 which is in the catalytic domain of the human protein, that is comparable to R878 in the murine protein, has been observed to suppress catalytic activity and appears to decrease binding to DNA [40]. In Figure 5 we showed the incidence rate in adult patients with DNMT3A mutations, it have been found in 14–34% of the cases of AML [41]. And around 5–15% of the cases of myelodysplastic syndrome [42], 10% of the cases of chronic myelomonocytic leukemia [43], 5.7% of the cases of primary myelofibrosis [44], 12% of the cases of systemic mastocytosi [45], and 18% of the cases of T-cell acute-lymphoblastic leukemia (Figure 5) [46]. Patients with acute myeloid leukemia due to repeated DNMT3A mutations have been found to have poor survival rate. This was shown to be independent of the following factors: 1-age; 2- the type of mutation; or the genetic location of the mutation; 3- the existence of tyrosine-protein kinase (FLT3) or nucleophosmin (NPM1) mutations; [18]. Moreover, it has been found that adult patients with AML are more susceptible to DNMT3A mutations, with most studies recording a DNMT3A mutation recurrence of 20–25% in de novo disease [18,47-49]. Mutation at the R882 position is also the most common type of mutation in other hematological malignancies, including myelodysplastic syndrome, chronic myelomonocytic leukemia, and myeloproliferative neoplasms, although this mutation tends to occur less frequently compared with that of AML[43,50]. One of the hematological malignances most frequently associated with mutation of DNMT3A is T-lymphoid malignancies, even though T-lymphoid cells have a more diverse domain distribution than the myeloid lineage and having 20% less affect at the R882 position [21,51,52]. An identical ratio of mutations at the R882 position has been identified in T-cell acute-lymphoblastic leukemia [46,53]. Inactivation of DNMT3A during hematopoietic stem-cell differentiation has been observed to promote CD8-positive peripheral T-cell lymphomas and chronic lymphocytic leukemia in genetically engineered mouse model [54].
Cancer is the main cause of death worldwide, and in 2018 alone accounted for 9.6 million deaths. The most frequently affected organs in terms of fatal malignancies are lungs (1.76 million deaths), colons (862 000 deaths), stomachs (783 000 deaths), livers (782 000 deaths), and breasts (627 000 deaths) [55]. In all cases, cancer involves a change in the structure of DNA that causes an alteration of the normal DNA regulatory mechanisms. Abnormalities in DNMT1, DNMT3A and DNMT3B that are involved in the expression of genes related to cancer have been observed in many different types of malignancy [2]. Epigenetic alterations might be a tool for the prediction of the clinical consequences [56]. For example, decrease activity of H3K4me2 is linked with poor prediction in prostate, lung and kidney cancers, although decrease activity of H3K18ac and H3K9me estimate a worse prognostic in kidney and lung cancer. However, the expression of higher activity of H3K9ac in patients with lung cancer is linked with a lower survival. Specific manner of H3K9me are linked with certain clinical consequences in acute myeloid leukemia [57]. Deviation of methylation is one of the role that would cause diseases, that involved cancer. De novo methylation is mediated by the de novo methyltransferases, DNMT1, DNMT3A and DNMT3B; DNMT3B has been demonstrated to be involved in advancing disease via silencing of tumor-suppressor genes [58]. Although it has been shown that DNMT3A plays a magnificent role in the induction and advancement of tumor development, however it does not appear to be involved in tumor initiation [58]. DNMT1 is predominately engaged in controlling cell development [59,60], therefore mutation in DNMT1 will lead to uncontrolled cell development and eventually cancer. Recent studies have shown that mutation, methylation-mediated by gene silencing often causes tumors [58]. Deletion of DNMT3A remarkably promotes tumor development and progression in a mouse model of lung cancer [61]. As previously mentioned, the DNMT3A gene functions in de novo methylation during embryogenesis and imprint establishment and repression, and a high rate of mutations of this gene can lead to colorectal cancer, breast cancer, ovarian cancer, esophageal squamous-cell carcinoma, hepatocellular carcinoma and pancreatic cancer [3,61-65]. DNA methyl ation plays major roles in the development of gastric cancer and the expressions of DNMT1, DNMT3A, and DNMT3B have been examined in 307 patients with gastric cancer [66 ]. The overall distribution of DNMT3A mutations in different types of cancer is summarized in Table 1. Furthermore, in normal human tissues, overexpression of DNMT3A has been detected in different types of cancer, such as prostate [67], pancreatic [32], and liver cancer [68]. Patients with papillary-thyroid carcinoma and follicular-thyroid carcinoma were shown to exhibit highly remarkable frequencies of DNMT3A mutations, which indicate the potential value of these mutations in guiding treatment of patients with thyroid cancer [69]. Another in vitro study showed increased levels of some DNMTs have been observed in ovarian cancer cell lines and primary ovarian cancerous tissues as compared to normal ovarian cells [2].
The power of the DNMT and its ability to be used as therapeutic tool were mentioned in many studies. It has been reported that DNMT3A gene could act as tumor suppressor and could be an important factor of lung-cancer malignancy [62]. It was accordingly found that the expression of DNMT3A can serve as an independent indicator for gastric cancer prognosis and may play an important role in gastric carcinogenesis [65]. The use of DNMT3A as diagnostic tool and a cancer indicator and biomarker is adopted in oncology centers. Screening for DNMT3A mutations can provide early detection for cancer, which will in return increase the survival rate and decrees the morbidity. The high expression of DNMT3A mutations in patients with papillary-thyroid carcinoma and follicular-thyroid carcinoma favor the screening and detection and as we mentioned before accelerate the healing process. The highest range of DNMT3a, and DNMT3b overexpression were also shown in-patient with breast cancer, 30% of patients revealed overexpression of DNMT3b in the tumor tissues compared to normal breast tissue [71]. Some studies suggest that patients with AML DNMT3A mutations will have better outcomes for have correlated with intensified treatment with DNA-damaging anthracycline therapy [72,73]. Also, it was shown that DNMT3A has recently emerged as one of the most important tumor suppressors in haematological malignancies. Its exceptional role is rooted in its crucial function in stem cells, in which it enables the first steps of haematopoietic differentiation. All the DNMTs studies on different kind of cancer indicate that DNMT3A are considered valuable targets for the design of specific anti-cancer strategies. More studies and clinical trial should be conducted to show the actual effect on human target and the side effect that might be caused. Also the effects of DNMT aberrations in the promotion of tumorigenesis are not entirely clear, future work should be done more in this field.
In this review, we have sought to present an overview of the relationship between the DNA methyltransferase DNMT3A and cancer. DNMT3A mutations have been found to be associated with many types of hematological malignancy. DNA methylation is an epigenetic alteration that is critical in development, imprinting, stem-cell management, and X-chromosome inactivation. DNMTs are classified into three subcategories, namely DNMT1, DNMT2, and DNMT3, all of which have catalytic activities. Cancer occurs when there is a loss of control of cell division and involves a change in DNA structure that causes an alteration in normal DNA regulatory mechanisms. DNMT3A and DNMT3B are involved in DNA methylation and mutations of DNMT3A have been identified to play a role in the development of hematological neoplasms. Further studies characterizing the DNMT3A protein, along with basic research and clinical data, will produce new insights that will hopefully lead to the development of new therapeutic strategies.
This research received no external funding.
The authors thank King Fahad Medical City (KFMC).
The authors report no conflict of interest.