The Dilemma of Metastatic Axillary Cancer versus Primary Ectopic Breast Cancer
The clinical presentation of a new axillary mass is frequently due to metastatic malignancy such as breast or other diseases such as lymphoma. A recent case of a patient presenting with a large axillary mass was initially considered to be a possible metastatic lesion with a focus on finding a primary through exhaustive investigations including expensive imaging modalities such as CT scan, PET/CT scan and MRI. Finally, core-needle biopsy of the mass yielded a diagnosis of invasive ductal carcinoma with the possibility of the lesion being either a metastatic breast carcinoma or the relatively rare lesion of primary ectopic breast cancer arising in the axilla.
Our case presentation to follow demonstrates the challenges of making a diagnosis under these circumstances highlighting early consideration of a primary ectopic axillary breast cancer as a possibility and discussing features suggestive of ectopic axillary breast cancer that would lead to an early correct diagnosis and treatment.
Keywords: Accessory Breast Tissue; Carcinoma of Ectopic Breast TissueEctopic breast tissue develops during embryogenesis where there is a failure of resolution of the mammary ridges. The incidence has been reported to be 2% to 6% of females [1,2]. It can develop along the thoracoabdominal portion of the milk ridge which anatomically extends from the axilla down to the inguinal region. They may also be situated outside of the ridge, such as the face, neck, chest and back [3-5].
Primary carcinoma in ectopic breast tissue is very rare and accounts for only 0.3% of all breast cancers [1,2,4]. The ectopic breast tissue has also been found to have a higher propensity to develop malignancy and has an earlier age of presentation [2-4].
These tumours often occur close to normal breast and the axillary area which is the most common. Although rare, it is an important differential diagnosis when evaluating patients who present with lesions in the axillary region. The case description below highlights important aspects in the workup of a patient with an axillary mass along with the dilemma that occurs when differentiating a primary carcinoma of ectopic breast tissue in the axilla from metastatic axillary carcinoma. Accurate diagnosis has great importance in therapeutics and prognosis.
A 59-year-old post-menopausal nulliparous woman presented to our institution with complaints of a painless progressively enlarging right axillary mass for 4 months. She discovered this lesion which was 2 cm of diameter, one year earlier which had initially maintained a stable clinical appearance, without palpable masses in either breast. No treatment measure was taken initially. The mass had been increasing rapidly in size 4 months prior to the patient’s arrival in our institution. Her past medical history was significant for chronic hypertension which was managed well with medical therapy. She had no prior breast complaints or breast surgeries. Her family history demonstrated a strong predisposition to breast cancer with 2 of her 3 sisters having been diagnosed with breast cancer before the age of 40. As a result, she underwent a 30 gene panel test which was negative for any genetic mutations including BRCA 1 and 2.
Clinical examination revealed a 3 x 2 cm mass within the axilla with the presence of bilateral axillary breast tissue. The mass was found to be irregular, firm and mobile. No axillary adenopathy was palpated at the time of presentation and no mass was evident in either breast.
Prior to seeing us, she had high resolution ultrasonogram and Magnetic Resonance Imaging (MRI) which demonstrated normal breast parenchyma with no focal masses and a suspicious (BIRADS 4) 3 cm right axillary mass within the axillary tissue. Because clinical and radiological imaging of both breasts was normal, a non-breast primary was considered.
As a result, both Computed Tomography (CT) as well as Positron Emission Tomography (PET) Scans was ordered to establish a possible primary source for the axillary lesion.
PET/CT Scan showed FDG-avid focus in the right axilla but no active lesions elsewhere. The CT scan of the bone, chest, abdomen, pelvis and brain similarly showed no lesions outside the axilla. Ultrasound, MRI and PET/ CT also failed to demonstrate any further abnormal axillary lesions.
Eventually, consideration was given as to possibility of a primary breast carcinoma arising in ectopic axillary breast tissue.
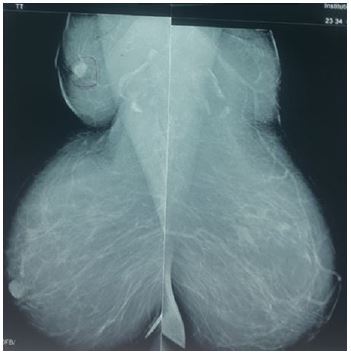
After careful inspection of the mammogram, a mass was seen in the right axilla along with continuity of the breast vasculature into the axilla (Figure 1). This suggests ectopic breast tissue as opposed to a metastatic axillary lymph node or other axillary lesion.
The patient underwent a core-needle biopsy of this axillary mass. The histology showed fibro-adipose tissue extensively infiltrated with ductal adenocarcinoma as well as lymphoid tissue with lympho-vascular permeation of tumour seen. Hence, the biopsy showed invasive ductal adenocarcinoma but failed to distinguish whether this was a metastatic axillary lymph node or a primary ectopic breast cancer.
The patient was then counselled on her surgical options and the case was discussed at our weekly multi-disciplinary team meeting. As a result of the ambiguous nature of her disease, the decision was made to proceed with wide local excision of the lesion (including the remainder of the axillary breast tissue) with excision of any adjacent palpable nodes on the right side.
She was evaluated by our anaesthetic team and was cleared for surgery. She tolerated the procedure well and her post-operative recovery period was uneventful. Thromboembolic prophylaxis with compression stockings were provided throughout the operative period. She was discharged the following day after surgery.
Postoperative histology confirmed that the lesion was a primary invasive grade 3 ductal carcinoma of mammary origin. The tumour size (T) was 2.5 x 2cm. All surgical margins were free of tumour. Immuno-histochemical studies showed positive estrogen and progesterone receptors and negative HER2/neu oncoprotein expression. Angio-lymphatic invasion was identified and 3 of 15 resected axillary lymph nodes (N) showed metastasis of ductal adenocarcinoma.
Her final pathological TNM Staging was pT2 N1 M0 (Stage IIB).
She received adjuvant chemotherapy with 4 cycles of Adriamycin/cyclophosphamide followed by 3 cycles of paclitaxel. The 4th dose of paclitaxel was deferred due to her development of bilateral pulmonary emboli after her 3rd dose. She was hospitalised and treated with anticoagulation therapy. A follow up CT scan of the chest showed no evidence of pulmonary metastasis.
The patient was also hospitalized for one episode of febrile neutropenia after the 1st cycle of paclitaxel and she was managed with intravenous antibiotics therapy. Granulocyte colony-stimulating factors were therefore given subsequently with cycles of chemotherapy.
Apart and separate from the issues discussed above, after her 4th cycle of chemotherapy, she developed a localised area of peau d’orange and an area of erythema in the peri-areolar area of the right breast. Our differential diagnosis at this time included post chemotherapy skin changes in the breast which has been described in the literature), inflammatory breast cancer and mastitis [7]. As a result, we counselled the patient on her diagnostic options which included a punch biopsy of the involved area.
However, at this time, she requested bilateral prophylactic mastectomies. This was a reasonable option because of her very strong family history of breast cancer, even though she was BRCA negative, recognizing that most hereditary breast cancer are not usually associated with the recognized gene mutations and that we had not completely ruled out a primary focus of carcinoma in the breast itself [8]. She underwent bilateral mastectomies with special consideration given for DVT prophylaxis including stockings and anticoagulation with heparin. Her post-operative recovery was similarly uneventful. The post-operative histology demonstrated normal histology of both breasts.
She has since completed radiotherapy to the right axilla and is on hormonal therapy with aromatase inhibitors. Our patient is currently asymptomatic and one year disease free.
Ectopic breast tissue (EBT) has been reported in up to 6% of the population, more commonly in the axilla [9]. Embryologically, EBT is formed once the mammary ridge does not regress normally during development. This may have various clinical presentations as described by Kajava in 1915 as the following:
Class I - complete breast including glandular tissue, nipple, and areola
Class II - glandular tissue and nipple, without areola
Class III - glandular tissue and areola, without nipple
Class IV - only glandular tissue
Class V (pseudomamma) - only nipple and areola, without glandular tissue
Class VI (polythelia) - only the nipple
Class VII (polythelia areolaris) - only the areola
Class VIII (polythelia pilosa) - only hair
It should be noted that “polymastia” refers to the presence of more than two breasts. It is clinically equivalent with the terms “ectopic”, “aberrant”, “accessory” and “supernumerary” breasts [10,11].
EBT can develop pathology that a normal breast would. This includes benign pathologies such as fibroadenomas, fibrocystic changes, mastitis and atypical hyperplasia both ductal and lobular. The tissue itself is hormonally active and as such, responds to hormonal stimulation especially during menstruation and pregnancy. Consequently, they may become engorged or may even lactate during these physiological states.
Malignant neoplasia such as adenocarcinoma of the breast can also occur in these patients. Carcinoma originating from EBT is very rare and is responsible for 0.3% of all breast cancers [12]. Most carcinomas of EBT occur in the axilla [13]. As with normal breast tissue, ductal adenocarcinoma is most commonly reported pathology. A review of the literature also describes other histological subtypes such as lobular and medullary [14-16].
The diagnosis and management of accessory breast carcinoma follows the same principles as that of normally situated breast tissue once the diagnosis of ectopic breast lesion is considered. As such, any palpable lesion in EBT should be subject to the same investigations. This includes triple assessment using clinical examination, imaging including ultrasonography and histological examination of the lesion using core-needle biopsy and fine-needle aspiration cytology. Standard mammograms will not necessarily demonstrate EBT due to its location. The differential diagnosis of a palpable lesion in EBT in the axilla includes primary carcinoma of mammary origin, metastatic carcinoma, lymphoma and hydradenitis suppurativa.
Once metastatic disease as well as simultaneous lesions in the breasts has been excluded, the treatment of choice is wide surgical resection of the primary tumour with axillary lymph node dissection. Mastectomy is not indicated unless clinical examination and imaging of the anatomic breast demonstrates disease. In our case, bilateral mastectomy was only performed due to the patient’s desire. In the context of risk reduction, she also underwent excision of the contralateral axillary breast tissue. Because we were not certain of an occult primary in the breast, the patient’s request for mastectomy was welcomed because it allowed us to confirm whether there was a carcinoma in the usually located breast tissue.
Some have recommended mastectomy of the ipsilateral breast if the dissected axillary lymph nodes show metastasis of carcinoma, but this does not result in a better prognosis [12,17,18]. A study of 90 cases of carcinoma of EBT origin concluded that there is no survival advantage of modified radical mastectomy over that of local excision combined with axillary dissection or radiation [19].
Postoperative treatment for carcinoma originating in EBT is the same as for non-ectopic breast cancer. This includes external radiotherapy to the tumour site for local control. It should be noted that radiation of the ipsilateral breast is not usually performed. EBT carcinoma has been associated with a higher and earlier incidence of axillary lymph node metastasis than normal breast tissue. As a result, these patients also commonly require systemic adjuvant chemotherapy and highlight the importance of radiotherapy to the axillary region. Hormonal therapy is also used if receptor positive.
Studies have demonstrated that EBT is more prone to malignant neoplasia than that of normal breast tissue [20]. EBT also carries a poorer prognosis than carcinoma originating in normally situated breasts [21]. The prognosis is thought to be poor because early diagnosis is difficult.
Our case presentation demonstrates that focussing on metastatic disease as the etiology of an axillary mass could lead to expensive unnecessary investigations. Although carcinoma arising in axillary breast tissue is a rare diagnosis, this possibility should be considered. Close assessment of the mammogram, looking for continuity of breast vasculature into the axillae would suggest ectopic axillary breast tissue as was the situation in our case presentation.
Prompt core-needle biopsy would give the diagnosis of breast origin when accompanied by immunohistochemistry leading to earlier diagnosis and treatment, but this alone does not rule out a possible primary in the non-ectopic breast tissue nor does it confirm whether the axillary lesion is primary ectopic breast carcinoma as opposed to a metastatic focus in the axilla. This has important diagnostic and therapeutic implications as outlined in our case report and review of the literature.