The Influence of Magnetic Field on Synthesis of Iron Nanoparticles
One of today’s tasks is the development of new strategies for the synthesis of ferromagnetic, superparamagnetic nanoparticles immobilized in a polymer or carbon nanostructured matrix, and studying of the possibility of influencing their structure and magnetic properties.
When the critical parameter that determines certain phenomena (the size of magnetic domains, the characteristic length of exchange interactions, the length of magnetostatic interactions, etc.) becomes comparable to the particle size, nanoscale magnetic materials exhibit sharp (dramatic) variability of magnetic properties. Therefore, there is a need to develop new methods of synthesis, protection and certification of such nanosystems to synthesize promising magnetic materials using technologically controlled processes.
The encapsulation of metal nanoparticles into carbon chemically inert graphite like shells allows the synthesis of new generation materials to begin. Magnetic nanoparticles occupy an important place among these materials and such nanoparticles, which can be technologically important magnetic materials, will be investigated in this paper.
The formation of nanocomposites, the production of nanoparticles of the same size and, accordingly, with reproducible properties, is one of the main technological tasks of this research. Methods of obtaining, which allow us to precisely control the size of particles are continuously improved. Magnetic nanostructured materials with unique electrical, mechanical, catalytic, optical and, moreover, specific magnetic properties, make it possible to constantly expand their fields of application in computer science, catalysis, for sensors production, in medicine and in biology.
The correlation between nanostructure and magnetic properties allows us to offer different types of magnetic nanostructures: (1) systems with isolated particles, whose unique magnetic properties derive from decreasing sizes of components; (2) ultrafine particles with a cortical structure; (3) materials in which magnetic interaction is the dominant property; (4) nanocomposites, which consist of magnetic particles encapsulated in a chemically inert matrix. The magnetic properties in this case are determined by the ratio of volume fractions of magnetic particles and the matrix.
In this work, we studied the effect of an external magnetic field on the formation and phase composition of iron particles synthesized in an arc discharge in a liquid phase. These products can be technologically important magnetic materials.
Keywords:Carbon Chemically Inert Graphite like Shells; Nanoparticles; Magnetic Field, Synthesis; Structure
Recently, nanoparticles (NPs) and nanostructures (NSs) have attracted close attention of researchers for their unique properties, potential applications, including new types of very active and selective catalysts, chemical and biological sensors, drug components, high-density media, magnet and optoelectronic devices, etc [1]. Most of the unusual properties of NPs are due to their high ratio of surface area to volume. The ability to control the value of specific surface of NP and its chemical composition opens up the possibility of controlling their properties and functional features. Surface phenomena were mainly studied in thin films and, to a lesser extent, in nanoparticles and materials based on nanoparticles.
The formation of nanocomposites, the production of particles of the same size and reproducible properties, is the main technological task for today. The search for synthesis methods that allow us to precisely control the particle size is continuous. Some methods that make it possible to obtain composites of polymers with nanoparticles already exist.
When the critical parameter that determines certain phenomena (the size of magnetic domains, the characteristic length of exchange interactions, the length of magnetostatic interactions, etc.) becomes comparable to particles size, nanoscale magnetic materials exhibit a sharp (dramatic) change in magnetic properties. Therefore, there is a need to develop new strategies for the synthesis and certification of such systems to produce promising magnetic nanomaterials in a controlled manner.
The correlation between nanostructure and magnetic properties allows us to offer various types of magnetic nanostructures [2]: (I) systems with isolated particles, whose unique magnetic properties result from the decreasing sizes of the components; (II) ultra-thin particles with a cortical structure; (III) nanocrystalline materials, in which a substantial part of the volume is grain boundaries and intergranular spaces (interfaces) materials, in which magnetic interactions are the dominant property; (IV) nanocomposites consisting of magnetic particles directed onto a chemically inert matrix. The magnetic properties in this case are determined by the ratio of volume fractions of magnetic particles and the matrix.
The strategy of preparing composites containing ferromagnetic nanoparticles should be based on methods that allow not only to obtain stable products with high yield, but also the ability to control the particles size, their size distribution and interparticle intervals, etc. For the production of magnetic nanocomposites, such methods are widely used as the introduction of metal ions into a polymer matrix, the metallization of polymer surface, the evaporation of metal atoms, the ultrasonic method, a homogeneous molecular assembly, etc.
For example, nanocomposites containing Fe3O4 nanoparticles and g-Fe2O3 (£ 20 nm) were obtained by the exchange reaction of iron salts with perfluorin on ion-mine membranes (Nafion) by alkaline hydrolysis [3]. Ultrasonic decomposition of Fe(CO)5 was carried out in the presence of various surface-active substances, including polyvinylamine [4]. Covered Fe2O3 nanoparticles had a diameter of 5–16 nm. In another method [5], C2+ ions adsorbed in an ion exchange resin were reduced by excess NaBH4. Nanocomposites of a Co-B alloy with a wide particle size distribution (3–30 nm) were obtained.
The same approach was used to reduce cobalt salts in a solution of polyvinylpyridine [6]. The coercive force of a cobalt-containing polystyrene nanocomposite after low-temperature annealing (up to 720 K) grew up to 5 times [7].
According to another method, carbonyl co2(CO)8, embedded in the matrix of copolymer-styrene with DFA or isopropanol solution, can also be obtained after annealing at 470 K. Spherical cobalt nanoparticles with an average diameter of 26 nm were obtained by this method [8]. Magnetic properties were also found in Ni-polyethylene and Co-polyethylene nanocomposites. The conditions for their preparation were also given [9]. Although the methods of synthesis of such nanocomposites are well known, most of them have drawbacks such as a wide and shapeless particle size distribution, difficult to control composition and crystallinity of multicomponent systems.
PTherefore, the novelty of this work is in studying of self-organized metal-carbon nanoparticles, in which the synthesis of ferromagnetic nanoparticles and nanocarbon matrix occurs simultaneously in an external magnetic field.
It has already been proven that the composition of ferromagnetic nanoparticles, their size (5-30 nm) and size distribution as well as the thickness of a stabilizing polymer shell can be controlled at the stage of nanocomposite synthesis [10-15]. Previous studies have shown that nanocomposites of this type exhibit ferromagnetic properties at room temperature, with the coercive force depending on the pretreatment conditions. By changing the synthesis conditions (temperature, the ratio of the output reagents, the nature of the stabilizing matrix), nanoparticles of elemental metals, their oxides and carbides of the required size, shape and structure (for example, the inner shell) and with a uniform distribution in the matrix can be obtained [12]. It can be controlled in this way, and magnetic properties can be changed. In the case of bimetallic nanohybrid compositions (Fe-Pt, Fe-Co), the possibility of obtaining intermetallic compounds on a nano-scale was analyzed.
Magnetic nanoparticles for studying various limiting phenomena, including magnetic coupling through a nonmagnetic layer and anisotropic exchange effects (Co – CoO – Co, Co – Pd – Co compounds, Co – TiO2 – Co compounds), magnetic proximity effects (Co – Pd, Fe -Pd), exchange-jump effects (SmCo-Co, Nd-Fe-B-Fe3B) were also prepared by thermal decomposition of metal-containing compounds in a liquid (mineral oil or polymer solution in oil) [16-17].
Magnetic recording is the predominant technology for data storage, and further promises a rapid progressive increase in recording density. The goal of magnetic production to achieve a recording density of 1Tbit / in2 requires a further reduction in the size of magnetic particles to about 4 nm with an anisotropy that is strong enough not to change their magnetic moments during thermal vibrations. Particular attention was paid to Pt alloys with 3d metals for their high magnetocrystalline anisotropy. Alternative ways of synthesizing highly anisotropic isolated, self-organizing magnetic nanoparticles [18] and their magnetic stability [19] are currently under investigation.
One of the methods for producing highly dispersed ferromagnetic nanoparticles is the arc discharge method in the liquid phase (DLF) [20-21]. This method has a number of technological and physical advantages. It is quite simple in hardware design, has environmental cleanliness, waste-free technology, low energy consumption, economical. In addition, this method is characterized by high temperatures (~ 4000K) in the zone of nanoparticle synthesis, ultra-high cooling rates (~ 109K/s), and high dispersion of the resulting product (10-1000 nm).
The DLF method consists in the excitation of an electric discharge between two electrodes or pieces of material to be dispersed, placed in a dielectric liquid medium. Under the influence of a spark discharge, the microregions of electrodes or granules are melted and metallic vapor and molten droplets are released into the surrounding liquid (spark discharge zone), in which metal particles are formed during their rapid cooling.
With DLF method in area of plasma channel and in the surrounding liquid, electric and magnetic fields of complex configuration occur, which obviously influence the dispersion process. In this connection, the purpose of this work was to study the influence of an external constant magnetic field on the phase composition and dispersion of iron nanoparticles.
DLF were subjected to electrodes from steel 45, containing 0.66% by weight C according to X-ray elemental analysis oxygen and carbon atoms) on a device designed by authors of [ 21]. The schematic diagram of the installation is shown in Figure 1. The voltage between the electrodes was 300 V, the average current during the spark discharge was in the range of 200–300 mA. The crystal structure and phase composition of the composite was studied using a DRON-3.0 X-ray diffractometer in cobalt radiation, and the magnetic properties were studied using a ballistic magnetometer in the field range up to 800 kA/m at room temperature. An external magnetic field of 80 kA/m was formed using a permanent magnet, between the poles of which a reactor for the synthesis of powders was placed. Particle sizes were determined in three ways: by broadening lines in electron diffraction patterns (fine fraction), by electron micrographs (middle fraction), and by broadening lines on radiographs (large fraction) by the approximation method [22].
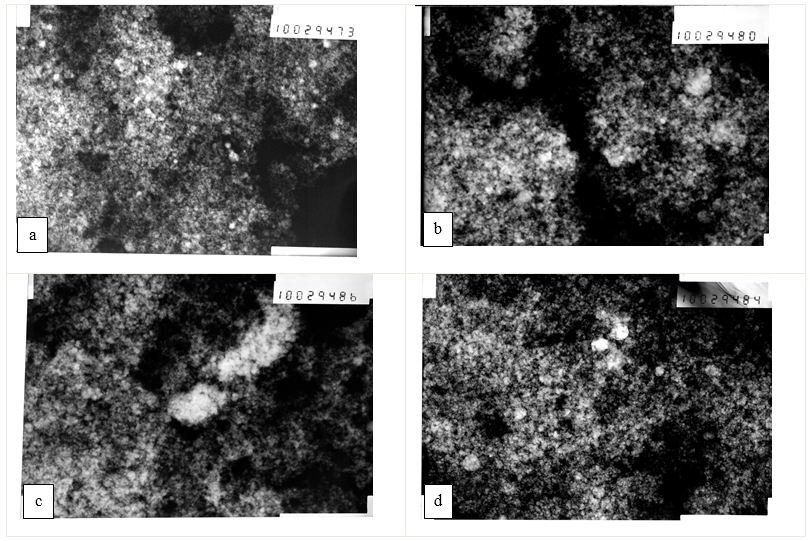
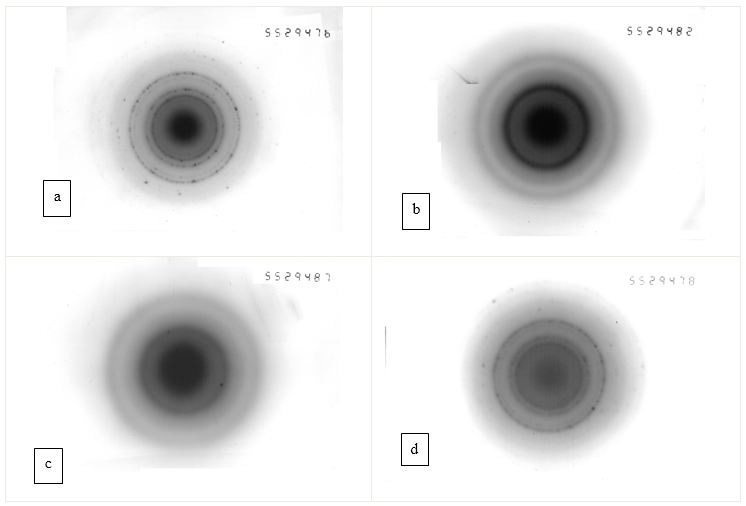
Figure 2 and Figure 3 show electron diffraction patterns and micrographs of highly dispersed Fe particles, obtained in distilled water and ethanol without a magnetic field and during its application. Micrographs show spherical particles with a wide particle size distribution. Using these micrographs, it is easy to determine the size of particles of the middle fraction, which, according to Table 1, are in the range of 200-300nm. The sizes of particles of the smallest fraction can be determined from the broadening of lines in the electron diffraction patterns, and the sizes of the particles of the largest fraction. That makes the dominant contribution to the X-ray scattering, from the broadening of the X-ray lines. The results of these calculations are also given in Table 1, and it indicates that the sizes of particles obtained by the DLF method are in a wide range of sizes from 20 nm to 1 μm. This conclusion is consistent with the results of studies by other authors, obtained in a number of works [20-21, 23-25, 26-33]. The experimental results shown in Figure 2 and Figure 3, as well as in Table 1, indicate that the application of a magnetic field has a noticeable effect on the dispersion of particles of only a small fraction, leaving the sizes of particles of medium and coarse fractions almost unchanged.
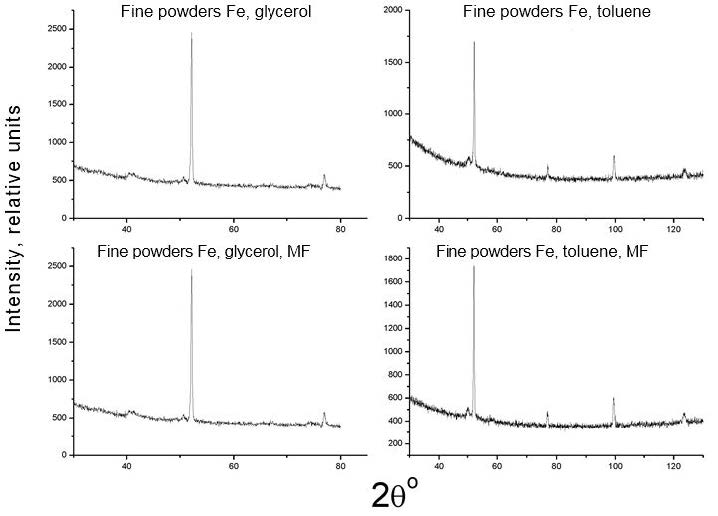
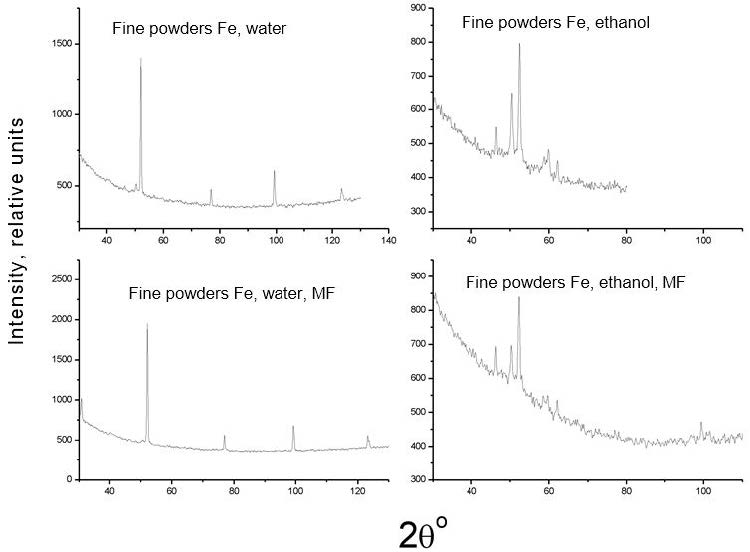
Figure 4 and Figure 5 shows the X-ray diffractograms of all investigated samples, both without overlapping and with the application of a magnetic field, and Table 1shows the results of determining their phase composition. These results indicate that the phase composition of the product obtained in various liquids, when a magnetic field is applied, behaves differently. Therefore, the phase composition of iron nanoparticles obtained in distilled water and ethanol varies only in terms of the percentage of observed phase components, the amount of less magnetic Fe3O4 oxide and γ-Fe when a magnetic field is applied, a few (~1 and 2%, respectively) decrease. For iron nanoparticles obtained in glycerol and toluene, both the quality and the number of observed phases change: graphite appears in glycerol when a magnetic field is applied, and the strong magnetic phase disappears in toluene α-Fe.
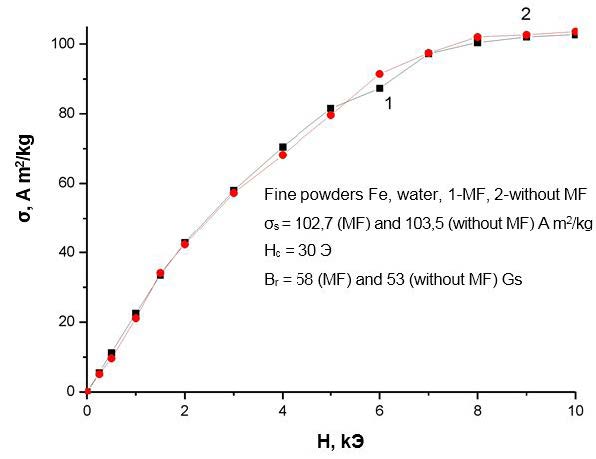
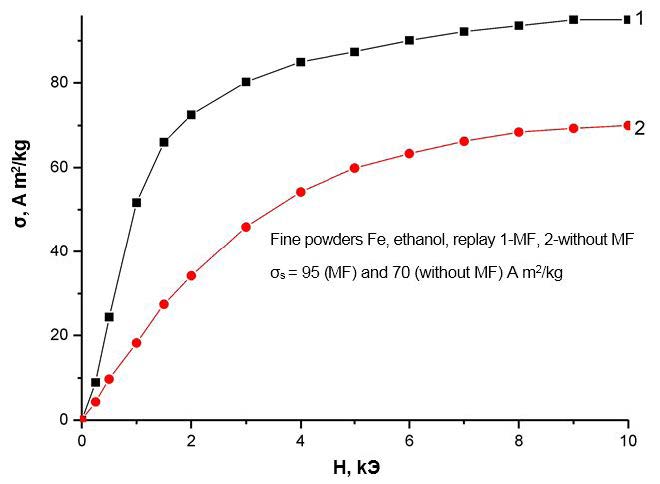
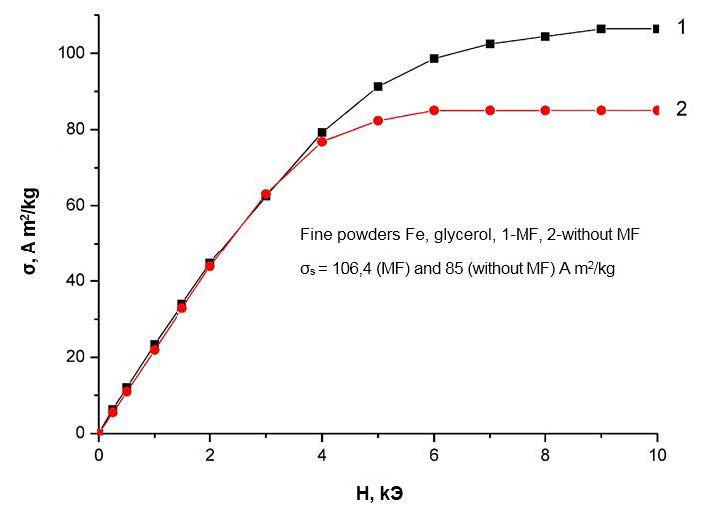
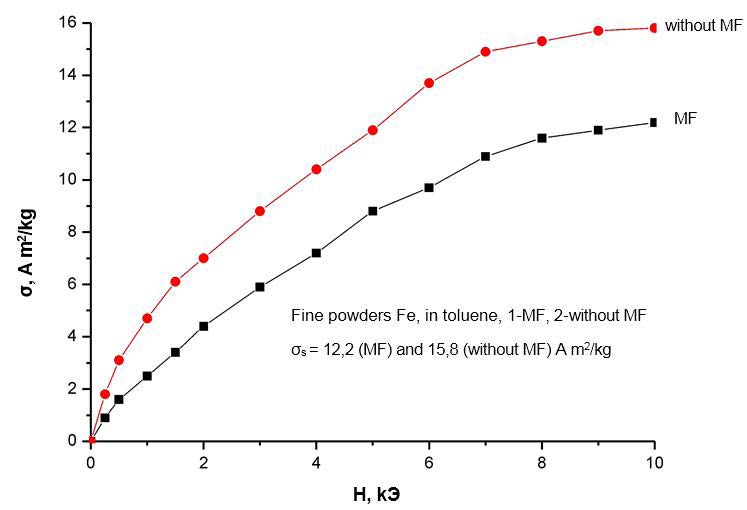
Even more significant changes occur when a magnetic field (MF) is applied for the field dependences of the specific magnetizations shown in Figure 6 ,Figure 7,Figure 8 and Figure 9. The first conclusion that can be made when analyzing these dependencies is that the field dependences also react differently to the application of a magnetic field for different liquids. Therefore, for iron nanoparticles obtained in water, the course of the field dependences of the specific magnetization practically does not change when an MF is applied. For nanoparticles of iron, obtained in ethanol and glycerin, the field dependences of the specific magnetization upon the imposition of MPs are higher than the analogous dependences measured without the application of MF, and for nanoparticles of iron, obtained in toluene, on the contrary, lower.
We now turn to the analysis and discussion of the experimental results obtained. Firstly, an increase in the number of more ferromagnetic α - phases in powders, as well as a decrease in the size of the critical nucleus during the application of MF, can apparently be explained by the negative contribution of the interaction energy of magnetic moments of particles and nuclei of the ferromagnetic phase to the total energy of the system. That leads to the growth of a thermodynamic stimulus for the formation and growth of these embryos. Indeed, when an MF is switched on with a voltage H, a particle with a magnetic moment M acquires energy -M×H cosφ (where φ is the angle between the direction of the magnetic moment of particle and the applied magnetic field), and now the overall balance of free energy ΔF during the formation of a spherical nucleus a ferromagnetic phase with radius r will look as follows [37,38]:
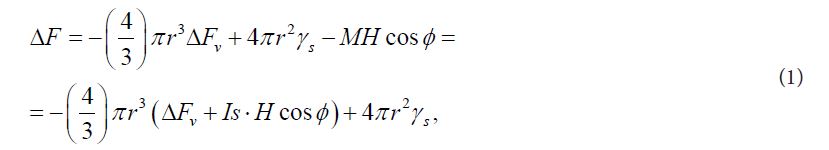
Where ΔFv – change of free energy due to the formation of a spherical nucleus of a new phase in zero MF, γs – specific surface energy, Іs – saturation magnetization of the embryo material. This leads to the fact that in the expression for the radius of the nucleus of critical sizes rс, an additional term appears:

That should lead to its decrease. This is the picture observed in our experiments. Indeed, the results of measuring the particle size of the smallest fraction, given in Table 1, testify to their decrease with the inclusion of MF.
The same reason can be explained by an increase in the amount of the strongly magnetic α-phase when an MF is applied. It can be shown that the additional term that arises in formula (1) due to the inclusion of MFs will also appear in the expression for the kinetics of nuclei growth of a strongly magnetic phase, which ultimately increases its total amount in the sample.
It should be noted, however, that a change in the phase composition of nanoparticles when an MF is applied cannot explain the observed change in all magnetic characteristics. For example, the imposition of MF in obtaining iron nanoparticles in ethanol leads to an increase in the amount of α-phase by 2%, while the specific saturation magnetization increases by almost 30% (Table 1 and Figure 7 ). A similar discrepancy is also observed for nanoparticles obtained in glycerol and toluene. On the other hand, the application of MFs in obtaining VDP in distilled water increases the amount of the α-phase in the nanoparticle by% by 1%, although this does not affect the magnitude of the specific saturation magnetization (and Figure 6). Additionally, the imposition of MF in obtaining nanoparticles in toluene leads to the opposite compared with nanoparticles obtained in ethanol and glycerin. The effect is that the specific saturation magnetization with the imposition of MF in this case does not increase, but decreases. This decrease can be partially explained by a corresponding (~ by 2%) decrease in the amount of the strongly magnetic α-phase. However, this decrease in the amount of phase does not fully explain the magnitude of the observed effect (~ 25%, and Figure 9), and, moreover, does not agree with the above arguments.
In conclusion, it should be noted that the magnitudes of the observed effects could probably be more significant when using external MFs of greater intensity. And yet, studies have shown that the use of external MFs can be used to refine mechanisms for formation of fine particles with DLF materials, and also as an additional factor for controlling the phase composition and dispersion of the powders obtained.
In conclusion, it should be noted that the magnitudes of the observed effects could probably be more significant when using external MFs of greater intensity. And yet, studies have shown that the use of external MFs can be used to refine mechanisms for formation of fine particles with DLF materials, and also as an additional factor for controlling the phase composition and dispersion of the powders obtained.