The Usage of Date Pits for Treatment of Thickened Sludge
Sludge often contains high concentrations of heavy metals as lead, mercury, cadmium which negatively affects the reuse of sludge after dewatering. Moreover, it affects the environment and the public health of the human. Date pits for sludge treatment have high degree of adsorption, natural, cheap and easy to obtain. In previous study, flotation technique succeeded for dewatering sludge and the best conditions were recorded. Optimum results were, bubbling pressure 0.6 bars, best Alum dose as coagulant 80 mg/L, and nitrogen gas economic dose is 60 mg/L. The dewatering efficiency was about 85%. In this research, samples of thickened sludge were first enriched with fixed dose of 2 mg/L of lead, and 2 mg/L of mercury. (There is no relationship between the percentage used and the real sample, but the purpose was to increase the amount of heavy metals to show the efficiency of the dates pits in the removal of heavy metals). Different doses of date pits then added to the sludge samples. The effectiveness of elimination heavy metals was about 85%. The outcomes demonstrate the achievability of utilizing date pits for elimination of heavy metal from sewage sludge.
Keywords: Sludge Treatment; Dewatering; Flotation; Date pits (DP)
The expansion in urbanization and industrialization has brought about a radical increment in the volume of wastewater and sludge produced around the world. Sewage sludge is frequently considered for use in agriculture because of the wealth of organic matter and nutrients [1]. This is anyway limited by the presence of conceivably unsafe constituents which include pathogenic organisms life forms, heavy metals, dissolvable salts and other follow constituents present in sewage sludge [2].
Sewage sludge is the solid, semi-solid or fluid residue produced amid the treatment of residential sewage completed in treatment works [2].
The disposal of Sewage Sludge (SS) or its application on farmlands is of general health concern, attributable to the possibilities of Heavy Metals (HMs) in SS to weaken soil, groundwater quality and bio-accumulate in food chains. Traditional inorganic chelating specialists utilized for eliminating HMs are for the most part costly and have negative environmental effect. Many reports have appeared on the development of low – cost adsorbents prepared from cheaper and readily available materials. Solid substance with large surface area, micro porous character and chemical nature of their surface have made them potential adsorbents for the removal of heavy metals from industrial waste water . A number of materials such as leaf mould, rice husk, groundnut husk, coconut husk and palm pressed fibers, coconut shell, coconut jute, coconut tree sawdust, cactus, olive stone cake and wool and pine needles (Table 1).
Lead: Lead is a naturally occurring bluish-gray metal. Although it can be found in all parts of our environment most of it is in human activities including manufacturing, mining, and burning fossil fuels. Lead is used in the production of batteries. Lead is probably the more common metal that is associated with heavy metal poisoning and toxicity. All the recent news about the presence of lead in kids’ toys has helped create public awareness about the dangerous effects of lead. Every year, industry produces about 2.5 million tons of lead throughout the world. Most of this lead is used for batteries [3].
Mercury: The organic form of mercury is more hazardous. The Environmental Protection Agency (EPA) has classified mercury chloride and methyl mercury as possible human carcinogens. The possibility of heavy metal reaching the waste water.
Heavy metals can collect in soil and in plants when sludge is applied as fertilizer and eventually can create destructive impacts in animals and people. Because of the high level of awareness familiarity with the negative effects of high grouping of heavy metals to the environment, stringent rules and verifications have been intended to application the utilization of sewage sludge to farming soils.
In this study, the adsorbent can be utilized as a low-cost condenser on the off chance that it requires low preparing, bounty in nature, secondary material or waste from another industry. Enormous measures of date pits are produced day by day (contribute to the search for less expensive absorbent materials and the potential for their use for various by-products of agricultural wastes that are often sources of pollution, therefore, date pits were used in this study. Sludge treatment is focused on reducing sludge weight and volume to reduce disposal costs, and on reducing potential health risks of disposal options. Water removal is the primary means of weight and volume reduction. The choice of a sludge treatment method depends on the volume of sludge generated, and comparison of treatment costs), making environmental issues and disposal. Thusly, the utilization of these wastes as a premium provides highly efficient technological means in dealing with heavy metal pollution and solving disposal issues, with insignificant speculation required. Consequently, there is a pressing need to consider every single potential source of cheap agriculture-based capacitors, and their practicality for heavy metal evacuation ought to be studied in detail.
That were used to characterize the date pits and their specie Date pits (DP) used in this study was collected from Damietta city in Egypt and washed using bid stilled water several times to remove surface adhered particles. Then it dried in (PS.3A, Advanced Technology) furnace at 150C for (24 h) up to stability of the weight. The dried date pits were crushed to give a dark brown powder with different diameter range. Separation of different date pits batches carried out by using (SAMA Sieve Shaker) and a batch with a mean diameter equal to 5 mm was used.
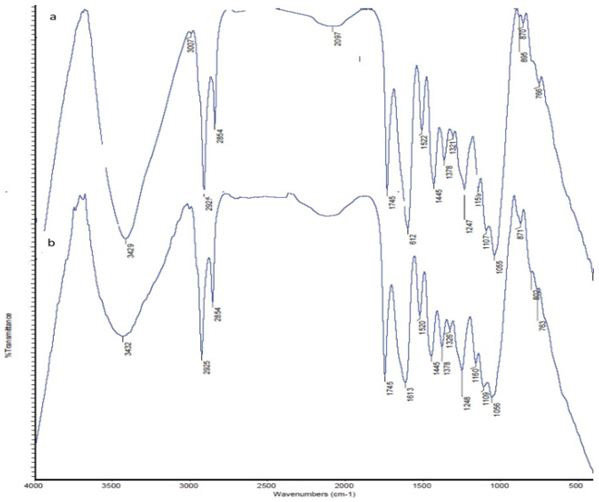
Figure 1 represents the Fourier transform infrared spectrum of the DP alone compared with the IR spectrum of the MB adsorbed species. The IR spectrum of DP showed strong bands at 3429, 3007, 2854, 1745, 1612, 1522, 1247, 1107.
The observed shift of bands in the IR spectrum to lower and/ or higher wave length as well as the appearance of new bands in the low frequency region (802 cm¡1) are most likely corresponding to the participation of the oxygen and /or nitrogen of the amino acids of the DP in adsorption process with the MB. The bands absence and the appearance of other bands is taken as a strong evidence for the adsorption process.
A potential value for drilling raw history as a cheap solid solvent. This work was presented to give basic data from the study of adsorption equilibrium and research in adsorption systems in Pb and Hg adsorption on raw date drilling. There is considerable interest in the spread of sediment in the agriculture area due to the ability to reuse important parts, for example, natural matter, nitrogen, phosphorus and other plant supplements [4]. However, due to the physical and chemical processes associated with wastewater treatment, the heavy metals available in wastewater are generally collected on the sludge that was created. Therefore, heavy metals are generally higher in the sludge than in the soil, where these components can be held indecisively in advanced soil layers. In this way, repeated application of sludge gradually increases the component of the soil. The large heavy metal in wastewater is restricted to its use as soil conditioners and organic fertilizers due to the high ability of the heavy metals to assemble in the food chain, soil quality, groundwater supply and thus human health and safety. Inorganic fermentation agents, which are the most known found in heavy metal extraction, have become extraordinarily productive because they build stable content with most of the large heavy metal. However, It’s bad signs, which include environmental sustainability, adverse health effects and prohibitive, have prevented its use. A wide different of extraction systems has been created to eliminate heavy metals from sludge sewage [5].
Toxicological studies have shown that some essential and non-essential elements become toxic at certain level of concentration. Due to environmental restrictions and authoritative limitations, water quality monitoring in process effluents has attained high national and international priority, especially for heavy metals. The most recent decades have seen an upsurge of benefit for the utilization of fluid-solid segregation, pre concentration and subsequent determination of some lethal follow metal species. The goal of the present work is to utilize new solid sorbent, date pits (DP) for the detachment and simultaneous determination of a few contaminations.
A new type of natural material (date pits) is used for the separation and pre concentration of many metal ion traces prior to their determination. Characterization of the date pits and their species with the studied metal ions is carried out using different techniques. The date palm, Phoenix dactylifera, is the oldest tree known to be cultivated by man. It is weal adapted to the dry and semi-dry regions of the world [4]. The date palm has been a significant source of date processing; it is known that the average weight of date pits ranges from 13-15% of the date weight Date pits were odorless and have light to dark brown color and a bland taste with slight bitterness [6].
Advanced procedures for treating wastewater containing heavy metals frequently include technologies for decrease of poisonous so as to fulfill innovation based treatment guidelines. This article audits the ongoing advancements and specialized pertinence of different treatments for the expulsion of heavy metals from wastewater. A particular focus is given to inventive physic-chemical removal procedures, for example, adsorption on new adsorbents, layer filtration, electrodialysis, and photocatalysis. Their focal points and limitations in application are assessed. The principle working conditions, for example, pH and treatment execution are displayed (Table 2).
For the three trees (Scotty and Shami, Bartender) minerals were determined from dates by Atomic Absorption Spectrophotometer.
The conceptual mechanism of heavy metal removal by chemical precipitation is presented in Equation 1.
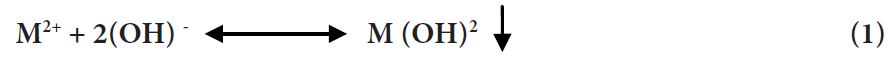
Where M2+ and OH− represent the dissolved metal particles and the precipitant, separately, while M (OH)2 is the insoluble metal hydroxide. Adjustment of pH to the essential conditions (pH 9–11) is the main parameter that altogether improves heavy metal evacuation chemical precipitation Adsorption on new adsorbents Sorption is ions of particles from water to the soil for example from solution phase to the solid phase. Chemical Precipitation Chemical precipitation is effective and cost-saving process for heavy metals removal from aqueous solutions and by far the most widely used process in industry because it is relatively simple to operate. In the precipitation process that can generate very fine particles that are held in suspension by electrostatic surface charges, chemical precipitants, coagulants, and flocculation are used to increase particle size through aggregation, resulting in production of large amount of sludge to be treated with great difficulties. However, this is usually effective to treat high concentration wastewater containing heavy metal ions [14].
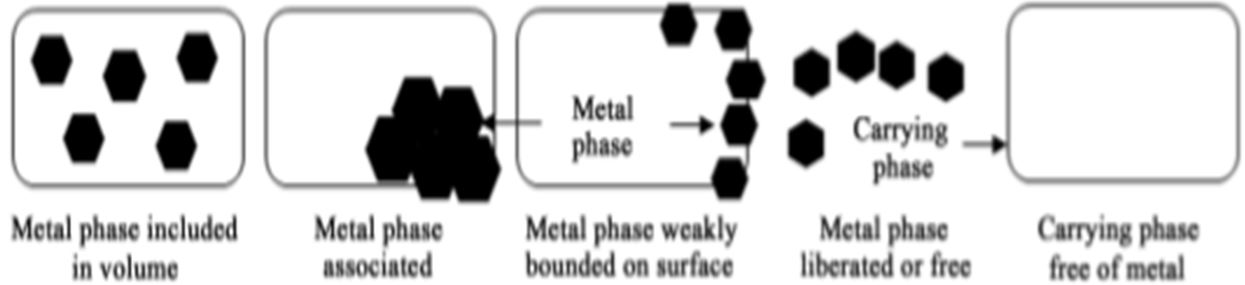
Hydroxide Precipitation The conventional chemical precipitation processes in which chemicals react with heavy metal ions to form insoluble precipitates include hydroxide precipitation and sulfide precipitation. The conceptual mechanism of heavy metal removal by hydroxide precipitation is presented in Figure 2.
Sorption actually describes a group of procedures, which includes adsorption and precipitation responses. Recently, adsorption has turned out to be one of the alternative treatment procedures for wastewater laden with heavy metals. Essentially, adsorption is a mass exchange process by which a substance is exchanged from the fluid stage to the surface of a solid, and becomes bound by physical and additionally chemical interaction [7].
Different low-cost adsorbents got from agricultural waste, industrial byproducts, natural materials, or adjusted polymers, have been developed and used to expulsion of heavy metals from mineral-contaminated wastewater. In general, there are three major steps in absorbing pollutants onto solids sorbent: (I) Transferring pollutants from bulk solution to absorbent surface. (ii) Adsorption on the particle surface. (iii) Transport inside the sorbent particle. Technical applicability and cost-effectiveness are key factors that assume significant roles in the determination of the most suitable adsorbents for inorganic waste treatment.
Chelation therapy is the use of chelating agents to detoxify poisonous metal agents, such as mercury, arsenic, and lead, by converting them to a chemically inert form that can be excreted without further interaction with the body [8].
The conventional methods include chemical precipitation, ultra filtration, ion exchange, reverse osmosis, electro winning, carbon adsorption and phytoremediation appear to be ineffective or extremely expensive or take long time for heavy metal ion removal from water and industrial waste water [9]. Efforts are presently being made to develop novel technologies that are low cost, eco-friendly and can efficiently remove the metal ions. Alternative technologies termed biosorption have been used in the last twenty years and are based on the metal sorption potential of certain natural and cheap biomasses like algae, fungi, bacteria and waste plant materials [13].
The biosorption can be defined as the ability of biological material to accumulate heavy metals from waste water through metabolically mediated or physicochemical pathways of uptake. The major advantages of biosorption over conventional treatment methods are low cost, high efficiency, minimization of chemicals, no additional nutrient requirement, and regeneration of biosorbent and possibility of metal recovery.
Heavy metal contamination has turned out to be a standout amongst the most genuine ecological issues today. The treatment of Heavy metals is of exceptional worry because of their complexity and persistence in the environment [1].
Heavy metals are commonly viewed as those whose exceed 5 g per cubic centimeter a substantial number of components fall into this classification, yet the ones recorded in Table 1 are those of significance in the environmental context. Arsenic is generally viewed a serious heavy metal despite the fact that it is really a semi-metal. Heavy metals cause genuine health impacts, including diminished development and low growth, cancer, organ harm, sensory system harm, and in extraordinary cases, death. Presentation to a few metals, for example, mercury and lead, can also lead to the development of autoimmune immunity, in which the person’s immune system attacks its own cells. This can prompt joint illnesses, for example, rheumatoid kidney disease, circulatory system, nervous system, and brain damage to the fetus. At higher portions, heavy metals can cause impermanent damage to the brain. Kids may get higher doses of minerals from food than adults because they consume more food for their weight than adults. Wastewater directions were built up to limit human and environmental presentation to hazardous chemicals (Table 3) [11].
The principle objective behind this paper is to research utilizing date pits for elimination of heavy metals from thickened sludge subsequent to dewatering. Date pits are more cheap adsorbents and their usage in many cases also outcomes for different agricultural waste results which are in many cases also contamination sources.
In previous study, we succeeded to introduce sludge dewatering using flotation by use of nitrogen gas, and alum doses with definite bubbling pressure. The relation between flotation time, height of sludge or separated liquor, pH, BOD5 and TSS values were observed and the best values of the doses were recorded. (Usually, conventional methods use air for flotation of the sludge, dissolved air flotation is a physical process for reducing the sludge volume and moisture content for dewatering sludge. As a unique and new idea, we introduced using N2 gas instead of air for sludge flotation and dewatering).
The location of the study was in Port Said sewage treatment plant, Port Said city. Sludge was collected from the facultative Pond. A comprehensive report of the characteristics of these raw samples was kindly produced by the laboratory chemists from Port Said city plant.
The characteristics and parameters of sewage sludge were collected on five random days (covering the period of study). It can be seen that the sewage under consideration is of medium strength. Contains a sufficient amount of organic matter and nutrients required for the life of organisms. It is noticed also that there is a considerable deviation in characteristics from day to day.
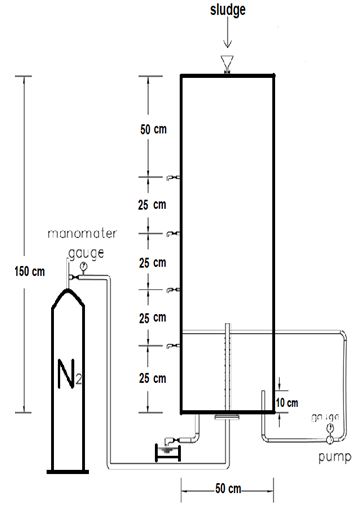
The model was manufactured in the sugar beet plant in Belqas village and then transferred to the sewage plant in Port Said city. The model body is made of a welded iron cylinder to withstand the internal pressure. Inside the pipe is a perforated pipe to distribute the gas inside the sample for ease of distribution. Nitrogen tube as a source of nitrogen gas used in the experiment shown in Figure 3 and 4.
The experimental model was positioned in an open area in Port Said sewage treatment plant, and was exposed to sunlight and wind through the day. The data presented in this study was obtained during six days period from 1/7/2017 to 6/7/2017.
In previous study, the experiment was conducted in three stages:
In previous research, investigation was carried out to use nitrogen gas with alum aid for flotation of sludge. The solids content of thickened sludge in flotation layer produced from biological treatment of domestic or industrial wastewater using dissolved nitrogen gas and alum doses in the sludge were succeeded [12]. The sludge was concentrated by 8-9 times after 20 minutes of flotation, with separation efficiency of 85%. The best conditions of in this study were obtained as, the bubbling pressure of nitrogen gas was 0.6 kg/cm2, the alum dose was 80 mg/L. and the nitrogen gas amount was 60 mg/L [12].
In this study, date pits will be used to try removing heavy metals from thickened sludge. The sludge sample was injected with constant doses, 2 mg/L of heavy metals (lead and mercury).
In order to examine the usefulness of date pits and to observe the best dosage, Dates pits were used in different doses 50 mg/L and 100 mg/l and 150 mg/l. ( Furthermore, it is imperative to determine the Pb and Hg concentration by a more precise technique, such as atomic absorption spectrometry).
The analyses were done utilizing similar conditions succeeded already [12]. Excess heavy metals are a toxic contaminant and the least amount of it causes extensive damage and countless diseases. The date pits are absorbent, environmentally friendly, cheap and easy to obtain.
Experiments were also conducted on the thickened sludge and therefore heavy metals were concentrated in the sludge and the amount of date pits would be determined on the thickened sludge.
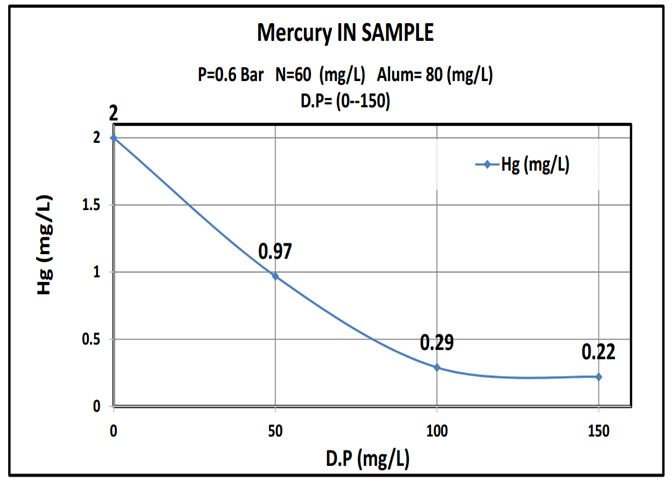
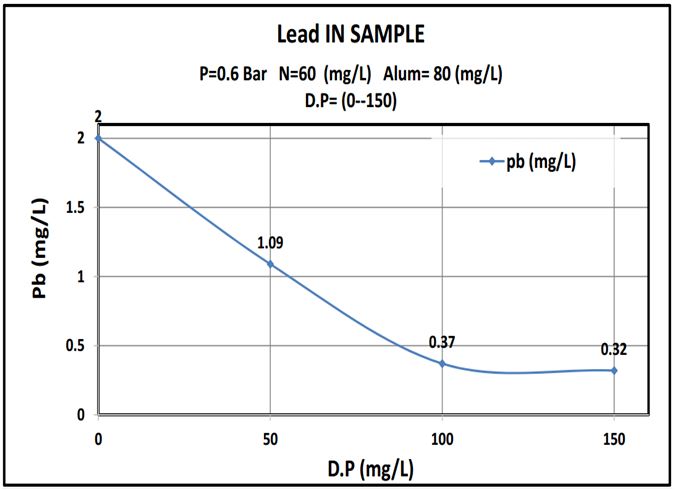
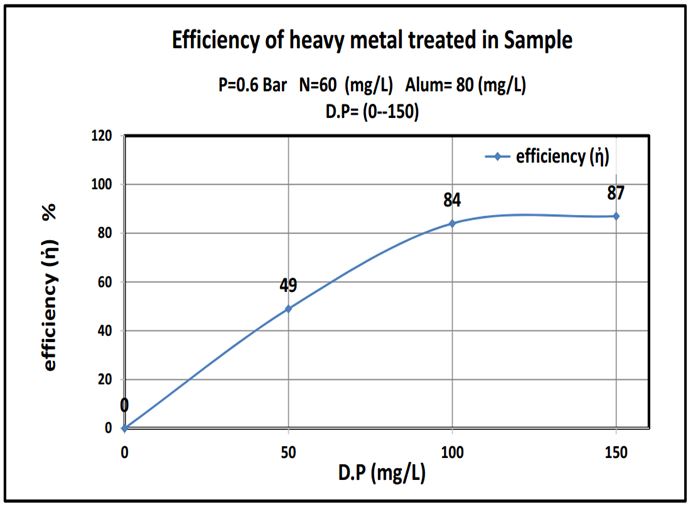
The outcomes were summarized in Table 4 and plotted as shown in curves (Figure 5, 6 and 7).:
According to the observed results and the previous discussion we can summarize the following:
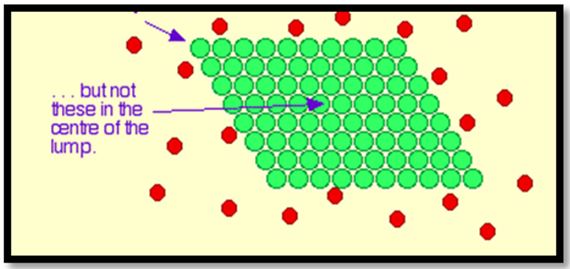
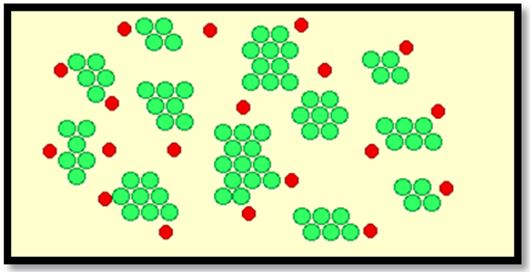
This work showed that date pits were a promising low cost adsorbent to be used in the removal of heavy metals and the results show that adding about 100mg/L date pits removed 85% of heavy metals, lead or mercury. (Where the reaction rate increases with the increase of the surface so it is a powder) The more finely divided the solid is, the faster the reaction happens. A powdered solid will normally produce a faster reaction than if the same mass is present as a single lump. The powdered solid has a greater surface area than the single lump (Figure 8 and 9).
Sewage sludge with low content of heavy metals can be reused as a beneficial source of date pits. At last, the outcomes demonstrate the possibility of utilizing date pits for expulsion of heavy metals from sewage sludge.
DP: Date Pits Pb: Lead Hg: MercuryConflict
The author confirms that this article content has no conflict of interest