Therapeutic Effects of the Application of a Novel Growth Factor Cocktail Solution (Moliniq™) Via Microneedling on the Scalp of Patients with Androgenetic Alopecia: A Split Study
Background: Growth factor cocktail (GFC) solution application via microneedling is being used as a successful and safe therapy for patients with androgenetic alopecia (AGA). However, additional studies on an effective GFC solution and a more efficient injection method are required.
Objective: This study aimed to determine the therapeutic effects of the injection of a novel GFC solution containing various fibroblast growth factors via microneedling in patients with AGA.
Methods: A total of 23 patients with AGA (12 men and 11 women) were enrolled in this study. Beginning with the first visit, the patients were treated six times over the course of 12 weeks at 2-week intervals. The scalp was divided into two parts (right and left); microneedling was performed at a depth of 0.9 mm with injection of normal saline on the right and 3.0 mL GFC solution on the left. To measure the treatment effect, we obtained clinical and phototrichogram images before the first treat- ment and at 12 weeks after the treatment.
Results: The phototrichogram images of the left scalp (control group) showed a significantly increased hair density from 154.9±28.5 to 175.1±32.8 hairs/cm2 and diameter from 55.8±8.4 to 58.7±9.3 µm (p<0.05). Meanwhile, the phototrichogram images of the right scalp (placebo group) showed a non-significantly increased hair density from 159.0±33.0 to 164.8±41.7 hairs/cm2; the diameter remained unchanged at 55.0±7.7 µm.
Conclusion: Novel GFC solution application via microneedling for 12 weeks was effective in treating AGA. However, further studies on the long-term efficacy of our novel GFC solution are needed to confirm the treatment effects and to define the treatment mechanism and the most effective microneedling depth.
Keywords: Androgenetic Alopecia; Moliniq™; Growth Factor Cocktail; Microneedle
Androgenetic alopecia (AGA) is the most common hair loss disease causing cosmetic and psychosocial problems [1-4]. Dihydrotestosterone, a potent form of testosterone, causes hair follicles to shrink, resulting in the transformation of terminal hair into vellus hair, which induces AGA [1,3,5-7]. Oral finasteride and dutasteride administration as well as topical minoxidil application have been the most common treatments for AGA to date [1-4,8]. However, it is important to note that the primary goal of treatment is to slow down or stop disease progression and prevent further hair thinning and that improvement may not always be possible [4,8]. Many studies on new treatments to replace traditional treatments are being actively conducted; however, there is still a lack of high-quality evidence [8].
As many studies on various factors contributing to hair growth and differentiation have been actively conducted in recent years, the application of a growth factor cocktail (GFC) solution via microneedling has been used as a successful and safe therapy for pa- tients with AGA [9-18]. Thus far, we have conducted several studies on the composition and injection method of GFC solutions to determine the proper treatment process [12-18]. In previous studies, several patients complained of discomfort owing to bleeding and pain during the procedure [13]. In this study, the treatment effect of the injection of a novel GFC solution at a depth of 0.9 mm using a microneedle and whether the pain that could occur is minimized were investigated.
The study included 12 men and 11 women from the Alopecia Clinic of the Department of Dermatology at the Myongji Hospital. These patients were aged 26 to 57 years, with the men classified as having Hamilton–Norwood types III, III-Vertex, IV, and V and the women as having Ludwig types I and II. Patients with severe seborrheic dermatitis, infectious or severe inflammatory skin lesions, keloid dis- ease or collagen and elastic fiber disease, immunodeficiency disease, or psychiatric problems; those undergoing treatments that could lead to immunodeficiency; or those undergoing hair treatments within 12 months from the start of the study were excluded.
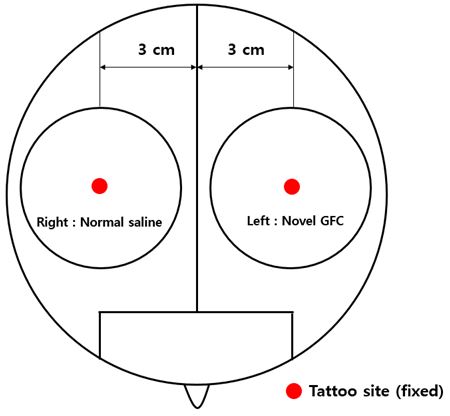
2Microneedling was used to treat all patients; no specific hair treatment was used. The scalp was divided into two halves on the right and left sides, with each injection site located at 3 cm from the central point. The right side was treated with normal saline and the left side with a novel GFC solution injected at a depth of 0.9 mm using a microneedle (Figure 1).
The GFC solution used in this study (Moliniq™; PnP Biopharm, Seoul, Korea) consisted of basic fibroblast growth factor (bFGF, 5 µg/mL), insulin-like growth factor 1 (IGF1, 5 µg/mL), vascular endothelial growth factor (VEGF, 5 µg/mL), stem cell factor (SCF, 5 µg/mL), keratinocyte growth factor (KGF) 1 (5 µg/mL), superoxide dismutase 1 (5 µg/mL), fibroblast growth factor 9 (FGF9, 5 µg/ mL), fibroblast growth factor 5-short (FGF5s, 10 µg/mL), and noggin peptide (500 µg/mL) (Table 1).
The microneedling device (Raffine®; Woorhi Mechatronics, Anyang, Korea) comprised nine 33-gauge microneedles that moved vertically (4200–6000 rpm), with a needle depth adjustment of 0.1 to 2.0 mm (Figure 2).
Before treatment, the novel GFC solution was dissolved in 3.0 mL of normal saline. Approximately 3.0 mL of the GFC solution was applied topically on the left side of the scalp and 3.0 mL of normal saline on the right side of the scalp (Figure 1). Microneedling (0.9 mm) was used on both sides, with a total treatment area of 5 cm2 for each location. For a period of 12 weeks, each patient received six treatments at 2-week intervals.
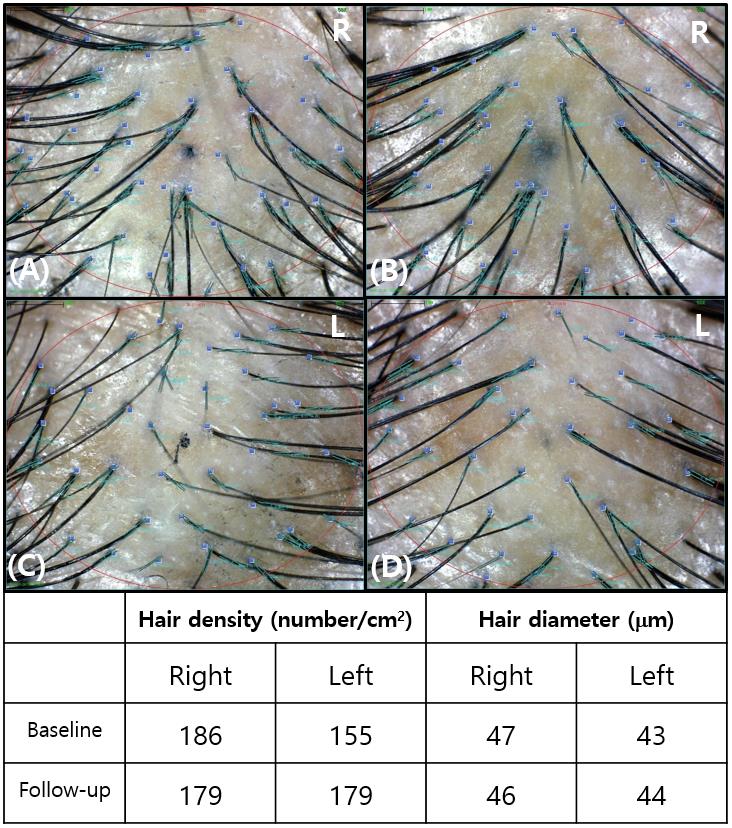
To compare the hair density and diameter, we tattooed the right and left injection sites 3 cm from the intended central point. On the tattooed areas, phototrichograms (Folliscope® 5.0; Lead M, Seoul, Korea) were performed immediately before treatment and at 12 weeks after treatment. Based on the phototrichogram images, an unbiased investigator blinded to the study information measured the number and diameter of hair.
All data were expressed as means ± standard deviations. The Wilcoxon signed-rank test was used to analyze the effectiveness of the treatment in the same patients using STATA/SE version 17 (StataCorp LP, College Station, TX, USA). This method assessed whether the difference following treatment was positive or negative by assessing this tendency. The statistical significance level was set at p<0.05.
Of the 24 patients, 23 patients aged 26 to 57 (mean, 40.6±10.9) years completed a total of six treatments every 2 weeks. This study population comprised 12 male patients with a mean age of 40.6±10.2 years and male pattern hair loss type III (n=3), type III-Ver- tex (n=2), type IV (n=2), and type V (n=5) according to the Hamilton–Norwood classification and 11 female patients with a mean age of 40.5±11.1 years and female pattern hair loss type I (n=8) and type II (n=3) according to the Ludwig scale (Table 2).
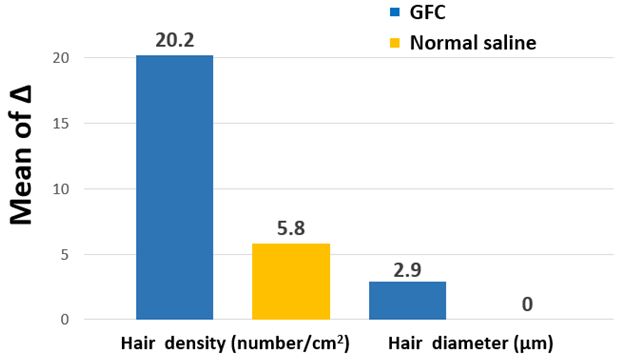
To compare the hair density and diameter, we obtained clinical and phototrichogram images before the first treatment and after the sixth treatment (Figure 3). The changes in the hair density and diameter between the right and left sides after treatment are shown in Table 3. Based on the phototrichogram images of the left scalp, the hair density increased from 154.9±28.5 to 175.1±32.8 hairs/cm2 (p=0.0003), and the hair diameter increased from 55.8±8.4 to 58.7±9.3 µm (p<0.0001). Based on the phototrichogram images of the right scalp, the hair density slightly increased from 159.0±33.0 to 164.8±41.7 hairs/cm2 (p=0.0683), while the hair diameter remained unchanged at 55.0±7.7 µm (p=0.6496). The hair density and diameter after 12 weeks of treatment with normal saline were not significantly different from those at baseline (Figure 4). However, as the composition of the novel GFC solution was changed, the odor generated during the procedure caused discomfort to both the clinicians and patients. In addition, mild pruritus and pain occurred, but did not significantly affect the patient’s quality of life.
Male and female pattern hair loss, also known as AGA, is characterized by non-scarring progressive size reduction of hair follicles, with a pattern distribution in predisposed men and women [1,2,8]. It can occur at any age, but is more common after puberty [19]. Male AGA is an androgen-dependent disease with hereditary predisposition, although the route of inheritance is unknown [8]. However, the role of androgens in female patients with AGA is still unclear [8]. In both men and women, AGA is not only a cosmetic issue but can also have a negative impact on patients’ quality of life, putting them at risk of depression, stress, and even suicide [19,20]. Currently, the treatments for AGA approved by the FDA include only minoxidil, finasteride, and low-level laser light therapy [1,4]. However, surgical methods, such as scalp skin auto-implantation and follicle autografting, as well as plate- let-rich plasma injections and microneedling, have been actively studied [1,2,8].
Some chemical signals that constitute the hair follicle cycle have recently been established [5,10]. They involve gene families, such as FGF, transforming growth factor-β (TGF-β), Wnt pathway, sonic hedgehog, neurotrophins, and homeobox, which are also found in other regenerative systems [5,10]. Among the various cytokines involved in the hair cycle, hair growth factors have been used to treat hair loss in many studies [10,12-18]. Several cases of AGA improvement have been reported in the literature with the use of GFC solutions injected using microneedles, and iontophoresis studies to improve GFC absorption have also been conducted [12-18].
Ocampo-Garza et al. introduced microneedling as an effective treatment for AGA [19]. According to the literature, microneedling has many benefits as a rapid, easy, and inexpensive procedure [11,19]. It can be used in combination with other procedures or medications; when coupled with minoxidil, growth factor, or platelet-rich plasma therapy, it has been shown to be helpful in indi- viduals with AGA [19]. However, there is no standard needle length; the needle should be long enough to penetrate the cutaneous barrier and maximize drug delivery or stimulation, while being short enough to cause minimal pain and skin damage [11,19]. Our previous study has shown that GFC treatment improves hair growth and diameter, with the effect varying depending on the microneedling depth. At a microneedling depth of 0.5 mm, we found that a solution consisting of IGF1, bFGF, VEGF, SCF, KGF2, noggin peptide, and SOD1 significantly improved the hair density and diameter [16]. In addition, a significant improvement was found when a GFC solution consisting of IGF1, stable aFGF, FGF5s, SCF, KGF2, FGF9, noggin peptide, protaetide, and NMN was administered at a depth of 0.8 mm [17]. However, in our previous study in which we used a depth of 1.2 mm, several patients stopped the procedure owing to severe bleeding and pain [13].
In this study, a GFC solution consisting of a total of nine growth factors (bFGF, IGF1, VEGF, SCF, KGF1, superoxide dismutase 1, FGF9, FGF5s, and noggin peptide) was used; bFGF, VEGF, and superoxide dismutase 1 were added as new factors, in contrast to previous studies (Cellcurex™) [13]. A wide range of FGFs, such as fibroblast factor receptor (FGFR) 1, FGFR2, FGFR3, FGFR4, FGF1, FGF2, FGF5, and KGF, are involved in the hair follicle cycle and growth and affect almost all follicle tissues, such as the papilla, matrix, inner root sheath, and outer root sheath [5]. IGF1 is known to increase the number of hair follicles and enhance the growth phase [14,22,23]. It prevents hair follicles from entering a catagen state by stimulating anagen-phase follicle cell prolif- eration and downregulating the expression of TGF-β1 in hair follicles in a dose-dependent manner [14,22,23]. VEGF is the most researched growth factor [23]. Dermal papillae, keratinocytes of the outer root sheath, and endothelial cells produce VEGF and have been shown to interact with many growth factors, including FGF2, and to play a role in angiogenesis [23]. In addition, SCF, noggin peptide, and SOD1 are also commonly used factors in hair growth procedures as ingredients of GFC solutions [12,15]. In this study, other growth factors were added to the GFC solution, and the depth of microneedling was set to 0.9 mm. Because Westerners generally have a thick scalp, it is better to set the microneedling depth up to 2.5 mm. Meanwhile, Asians have a rela- tively thin scalp; thus, the most appropriate microneedling depth was set to 0.9 mm in this study. Our analysis showed significant increases in both the number and diameter of hair in the patients with AGA.
In this study, the effectiveness of microneedling was observed in addition to that of the application of the novel GFC solution. In the right scalp, where microneedling was performed only with normal saline, no increase in the hair diameter was observed; how- ever, there was a slight increase in the number of hair. Although the p-value was 0.0683, this result can be meaningful when con- sidering the effectiveness of microneedling in several studies [4,8,12,19,21]. Nevertheless, the limitations of this study are the small number of enrolled participants and the short study period. Future studies with a similar group of participants but a sufficiently large sample size and a longer duration of more than a few months are needed to further assess the effectiveness of the treatment. Furthermore, microneedling may result in inaccuracies, depending on the clinician’s skills. In this study, the odor produced during the procedure was also uncomfortable for both the clinician and the patient. And mild pruritus and pain did not affect the patient’s quality of life. In our opinion, the application of our novel GFC solution via microneedling is an interesting treatment option for AGA with few side effects; therefore, it can be used in patients with severe side effects or poor compliance to traditional therapies.
In this study, the application of a novel GFC solution (Moliniq™) via microneedling for 12 weeks was found to be a successful treatment for increasing the hair density and diameter in patients with AGA. After the novel GFC (Moliniq™) treatment, both the number and diameter of hair increased significantly, and there were no side effects. However, further studies on the long-term efficacy of the novel GFC solution are needed to confirm more accurate treatment effects and to determine the exact treatment mechanism and the most effective microneedling depth.
There is no potential conflict of interest relevant to this article to declare.
This study was funded by the Technology Development Program (S2843228), supported by the Ministry of Small- and Medi- um-Sized Enterprises and Startups (Korea). This study (IRB No. 2021-09-006) is part of the clinical professor research program of Myongji Hospital.
MoliniqTM was provided by PNP Biopharm (Seoul, Korea).