Urinary β2-Microglobulin is a Sensitive Indicator for Renal Tubular Injury
Objective: After filtration through the glomeruli of the kidney, β2-microglobulin is reabsorbed by the renal proximal tubules. Increase in urinary β2-microglobulin indicates tubular dysfunction, and measurement of β2-microglobulin in urine sediment is useful to determine the source of kidney injury. CD133 has recently been characterized as a progenitor cell marker in the kidney, detecting injured epithelial cells in the proximal tubules. This study was designed to evaluate the correlation of increase in urinary β2-microglobulin and CD133 staining in patients with tubular injury.
Methods: Between 2009 and 2012, 47 patients with renal biopsies had prior RenalVysion™ analysis. Among these, 30 patients had elevated urinary β2-microglobulin, the remaining 17 had normal range of urinary β2-microglobulin. Immunohistochemical staining for CD133 was performed in the corresponding renal biopsy specimens. Confluent CD133 staining in proximal tubules was considered positive. The correlation of urinary β2-microglobulin and CD133 staining was evaluated.
Results: Of the 30 patients with elevated urinary β2-microglobulin, 26 showed positive CD133 staining in the proximal tubules. In 17 patients with normal urinary β2-microglobulin, 6 were positive for CD133 staining. Using positive CD133 staining as the end point, sensitivity was 86.6%, specificity was 64.7%, positive predictive value was 81.3%, and negative predictive value was 73.7%.
Conclusion: Measurement of β2-microglobulin in urine is a sensitive assay to detect tubular injury, and strongly correlated with CD133 staining in injured renal proximal tubules. Due to low specificity, patients with elevated level of β2-microglobulin in urine should be evaluated in conjunction with the clinical history and urine cytological analysis, as well as urine and serum chemistry.
Keywords: β2-microglobulin; CD133; Renal Biopsy; Tubular injury
β2-microglobulin is a single-chain, low molecular weight (MW=11.8 kDA) polypeptide [1,2] and has similar structure to the CH3 domain of the immunoglobulin molecule [3]. β2-microglobulin forms the invariant light chain portion of major histocompatibility complex (MHC) class I molecules [4-6], which can be found on the membrane of all nucleated cells [7]. Thus, cellular membrane turnover is the main source of serum β2-microglobulin [3]. Because of its small size, β2-microglobulin is filtered freely through the glomeruli of the kidney [8,9]. Then, a majority of β2-microglobulin in the filtrate is reabsorbed and catabolized by renal proximal tubular cells [8,10]. Only trace amounts of β2-microglobulin remain in urine and are excreted [10]. Therefore, β2-microglobulin serves as a useful biomarker to evaluate both glomerular and tubular function [11]. Elevated level of serum β2-microglobulin indicates glomeruli malfunction, while elevated urinary β2-microglobulin suggests tubular dysfunction [12]; the latter is associated with proximal tubule injuries due to a variety of causes such as viral infection, ischemia, and toxicity from medications or heavy metals [13,14]. Measurement of urinary β2-microglobulin has emerged as a popular method of assessing tubular function clinically. As such, measurement of β2-microglobulin in urine sediment is included in RenalVysion™ (Bostwick Laboratories, Uniondale, NY) as a biomarker to evaluate renal tubular function.
Although measurement of urinary â2-microglobulin is widely applied in current practice to detect tubular dysfunction [15,16], the correlation between increase in urinary â2-microglobulin and tubular injury has not yet been established at the molecular level due to lack of a reliable tubular injury marker.
Recently, CD133 has been shown to be a useful marker for detecting tubular injury [15,17]. CD133 is a penta-spanning transmembrane glycoprotein located on cellular protrusions and associated with cholesterol-driven membrane microdomains [18]. Normally, CD133-positive cells are one type of progenitor cell that is scattered along the tubular epithelium [12,17,19-21]. After tubular injury, CD133-positive cells proliferate and migrate to replace the neighboring dead cells, leading to flattened and elongated tubular epithelium seen in kidney biopsies [17,22]. Thus, confluent CD133-positive tubular cells in kidney biopsies, combined with morphologic features of tubular injury such as flattening and simplification of tubular epithelium, serve as a useful, reliable approach for identifying tubular injury.
The objective of this study was to examine the correlation between increase in urinary β2-microglobulin and tubular injury assessed by confluent positive CD133 staining in kidney biopsies. We found that measurement of urinary β2-microglobulin is a reliable and sensitive assay for screening of tubular injury.
Patients with RenalVysion™ analysis at Bostwick Laboratories between January 2009 and December 2012 were retrospectively reviewed. During this period, 5,494 patients had urinary β2-microglobulin measurements. Renal biopsies that contained less than 10 glomeruli per histological section or without enough tissue for immunohistochemical staining were excluded. Forty-seven patients had follow-up renal biopsies and were included in the present study.
Immunohistochemical staining was carried out using rabbit anti-CD133 antibody (1:50 Miltenyi Biotec Inc.) with archived unstained slides. In biopsies for which unstained slides were not available, additional new slides were prepared from remaining tissue stored in formalin-fixed paraffin-embedded blocks. All sections were cut at 2 μm.
Immunohistochemical staining for CD133 was conducted on paraffin slides using an Intelli-PathFLX ™ platform (Biocare Medical). Paraffin was removed with xylene, and tissue sections were rehydrated in graded alcohols. Endogenous peroxidase activity was eliminated by treatment of slides with 3% hydrogen peroxidase for 5 min. Antigen was recovered by placing tissue sections in a pressure cooker with Diva Decloaker (Biocare Medical) antigen retrieval solution (pH 6.2) for 25 min. Background Sniper (Biocare Medical) protein block was then applied for 10 min at room temperature. Antibody against CD133 was diluted to 1:50 with Da Vinci Green antibody diluent (Biocare Medical). Each section was incubated with anti-CD133 antibody for 30 min, and then treated by March 4 ™ horseradish peroxidase-labeled polymer-based detection system (Biocare Medical). The IP FLX DAB (Biocare Medical) was applied for 5 min. Slides were counterstained with diluted hematoxylin. Sections were dehydrated with graded ethanols and cleared with xylene.
Patient charts were retrospectively reviewed. Demographic information, including age and gender was recorded for each patient. Parameters of tubular injury, including serum creatinine, granular casts and cellular casts, as well as urine cytology findings were recorded. Staining with anti-CD133 was read by two pathologists who were blinded to the clinical data, final pathological diagnosis, and patient outcome. Confluent cytoplasm staining for CD133 with intensity stronger than background (1+) in proximal tubules was considered positive. Focal confluent positive CD133 staining was defined as less than or equal to 50% staining of proximal tubules while diffuse confluent positive CD133 staining was defined as confluent CD133 staining in more than 50% of proximal tubules in all renal biopsies. Scattered staining or no staining was considered negative. Staining in distal tubules was considered to be non-specific and was carefully excluded by reviewing the corresponding original histological slides as necessary.
Data were stratified according to urinary β2-microglobulin level as normal (< 300 μg/L) vs increased, to CD133 staining (positive or negative), and evaluated by 2 X 2 contingency table (Table 2). The sensitivity, specificity, predictive value for positive result and predictive value for negative result were expressed as follow:
Sensitivity = true positive/(true positive + false negative)
Specificity = true negative/(true negative + false positive)
Predictive value for positive result = true positive/(true positive + false positive)
Predictive value for negative result = true negative/(true negative + false negative)
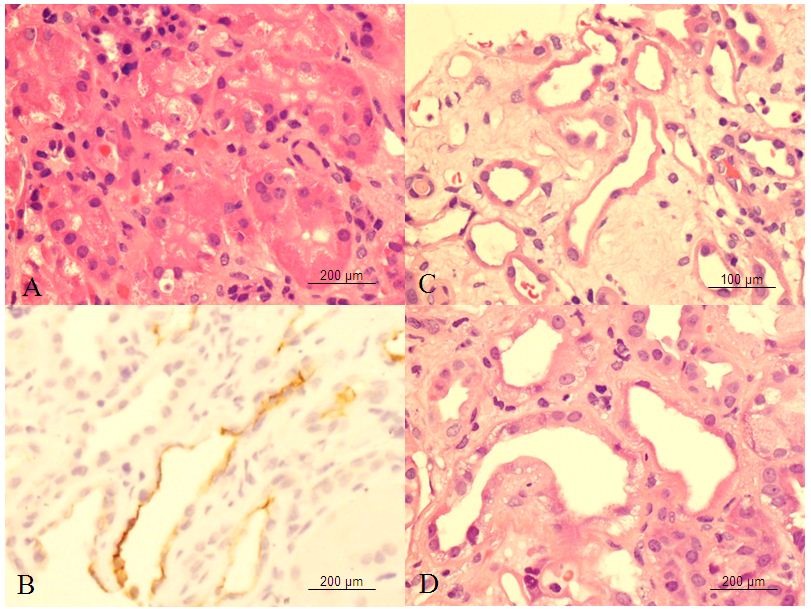
A total of 47 patients had urinary β2-microglobulin measurement and follow up renal biopsy. Thirty patients had increases in urinary β2-microglobulin; the remaining 17 had normal urinary β2-microglobulin. The demographic details for 26 patients (M:F = 17:9) with elevated urinary β2-microglobulin and positive CD133 staining in proximal tubules are shown in Table 1. The age of patients at the time of biopsy ranged from 16.0 to 83.0 years (mean, 54.4 ± 12.3 years). Renal biopsies were performed mainly in native kidneys but also in allograft kidney from 2 patients. Diagnoses in renal biopsies included a wide range of abnormalities, including glomerular and tubulo-interstitial diseases. These included acute interstitial nephritis, anti-neutrophil cytoplasmic antibody (ANCA) –associated glomerulonephritis, diabetic nephropathy, focal and segmental glomerulosclerosis, IgA nephropathy, and lupus nephritis. In addition, five biopsies showed tubular injury (Figure 1 C and D), two of which were toxic type of injury with cytoplasmic vacuoles Figure1 D). The remaining 3 biopsies were ischemia type of tubular injury.
Of the 26 patients with elevated urinary β2-microglobulin and positive CD133 staining in proximal tubules, 22 patients had increase in serum creatinine, ranging from 1.03 mg% to 6.0 mg% (Table 1). Granular casts were identified in 2 patients, who also had increase in serum creatinine. Increase in tubular epithelial cells was found in 13/26 (50%) patients in urine cytology. Eight of 26 patients also had microscopic hematuria.
The renal biopsies showed CD133 staining in parietal cells of Bowman’s capsule, which was used as the internal control. In the tubular system, distal tubules also showed cytoplasmic staining. In 30 biopsies with elevated urinary β2-microglobulin, 26 biopsies showed confluent CD133 staining in the apical cytoplasmic membranes of proximal tubules associated with evidence of mild to severe, overall mild flattening and simplification of the tubular epithelium (Figure 1 B). Among these, 22 biopsies showed focal confluent CD133 staining, and the remaining 4 showed diffuse confluent CD133 staining, 3 of which included tubular injury. Only scattered CD133-positive staining in tubular cells were identified in the remaining 4 biopsies, which were considered to be negative. In the 17 biopsies with normal urinary β2-microglobulin, 6 biopsies showed confluent CD133 staining in proximal tubules (5 are focal and 1 is diffuse). Scattered CD133-positive staining cells were seen in remaining 11 biopsies (Table 2). Using positive CD133 staining as the end point, sensitivity was 86.6%, specificity was 64.7%, positive predictive value was 81.3%, and negative predictive value was 73.3%.
We found that increased urinary β2-microglobulin strongly correlated with confluent CD133 staining in proximal tubules, corresponding with tubular injury. This indicates that measurement of urinary β2-microglobulin is a sensitive and reliable assay for detecting tubular injury. Measurement of urinary β2-microglobulin is simple, convenient and non-invasive [23]. With the high sensitivity (86.3%) shown in present study, this assay may be of value in clinical practice to screen for tubular injury in patients with acute kidney injury. Using CD133 as a tubular injury marker, we confirmed, at the molecular level the utility of urinary β2-microglobulin to detect tubular injury.
The clinical diagnosis of tubular injury is usually not difficult. The majority of patients with severe tubular injury do not require kidney biopsy to confirm the diagnosis and to initiate treatment. Glomerular disease is the major diagnosis in patients with kidney biopsy following increase in urinary β2-microglobulin (Table 1). Because urinary β2-microglobulin reflects proximal tubular injury [11], this finding suggests that, at least in part, many glomerular diseases are associated with tubular injury with varying severity. As serum creatinine is overall measurement of function of 4 compartments of kidney, increase in urinary β2-microglobulin in patients with elevated serum creatinine helps to determine the source of kidney injury. The present study also reveals that CD133 is a sensitive marker to detect tubular injury. Confluent CD133 staining was identified not only in the kidney biopsies with diffuse severe tubular injury but also in those kidney biopsies with mild and focal tubular injury (Figure 1 C). As showed in Table 1, many glomerular diseases were also associated with focal, mild tubular injury, and elevated urinary β2-microglobulin. Some were very focal and limited to a few proximal tubules. These focal lesions could be missed by routine light microscopy examination; however, they were identified by CD133 staining in the present study. In addition, confluent CD133 staining was also identified in 2 major types of tubular injury: ischemia and toxic type of tubular injury (Figure 1 B and C). As shown in Table 2, tubular injury was an additional diagnosis included in 5 of 26 biopsies (25%) (Table 1), 4 of which showed diffuse confluent CD133 staining in proximal tubules. These findings confirm that measurement of urinary β2-microglobulin correlates with positive CD133 staining in injured proximal tubules.
Specificity is relatively low in the present study. Six patients with normal urinary β2-microglobulin presented with confluent positive CD133 staining, which we considered false positive. This finding suggests that evaluation of patients with elevated urinary β2-microglobulin should be utilized in combination with the clinical history, (e.g., history of hypotension, recent history of medications use), increased in tubular cells in urine cytological analysis as well as serum creatinine. With RenalVysion™, the diagnosis of tubular injury is not only based on the level of β2-microglobulin in urine but also includes serum creatinine, presence of tubular casts, and cytological findings, such as an increase in number of sloughed tubular epithelial cells.
Several possible underlying causes might account for the false positive results in 6 biopsies. In general, the normal range of urinary β2-microglobulin is 230 μg/L to 300 μg/L [23]. RenalVysion™ is a urine-based test that combines urine chemistry and cytology evaluation to detect glomerular disease and tubular injury. The normal range for detecting tubular injury is set at < 300 μg/L in RenalVysion™. Among 6 false positive for CD133 staining biopsies, the amount of urinary β2-microglobulin in 2 patients were 280 μg/L and 290 μg/L, close to the upper limit of the normal range. The remaining 4 patients were found to have less than 210 μg/L, which is the measurement threshold of the assay. These findings suggests the “normal range” of urinary β2-microglobulin applied in RenalVysion™ could be reconsidered and perhaps lowered slightly. In addition, confluent CD133 positive staining was seen not only in injured proximal tubules but also in distal tubule, and atrophic tubules, all of which displayed similar morphological findings such as flattened tubular epithelium in kidney biopsies. Owing to limited sampling by biopsies, we cannot exclude the possibility of false-positive CD133 staining, especially in those biopsies with only focal tubular injury. If so, the actual sensitivity and specificity would be higher than in the present study. A tubular injury marker with specificity for proximal tubular injury, such as a kidney injury molecule, may be utilized in future studies to further define the normal limit of urinary β2-microglobulin and better characterize the clinical significance of urinary β2-microglobulin measurement.
Our study has multiple limitations. Only a small number of patients had follow-up renal biopsies after RenalVysion™ (47/5494, 0.08%). This finding suggests that RenalVysion™ can provide enough information to satisfy clinical management so that the kidney biopsy can be avoided in many patients. However, only a limited number of follow-up biopsies were available for the present study. A larger study cohort is needed to better correlate renal histopathology and immunohistochemistry in future study. Another limitation in the present study is the small tissue sample provided by kidney biopsy, which might be the cause of false negative in present study, especially in patients with focal tubular injury. Finally, the retrospective nature of the study may have introduced ascertainment and selection biases.
In summary, measurement of urinary β2-microglobulin is a convenient and sensitive approach to screen patients with kidney injury, primarily for patients with tubular injury but also those with glomerular disease. Patients with increased urinary β2-microglobulin should be evaluated in conjunction with the clinical history, urine cytological analysis, and urine and serum chemistry to determine the presence of tubular injury.
The authors would like to acknowledge the technical support from Etnita NuÑez in her professional performance of immunohistochemical stain.