W251 B6+ Gas Turbine Engine Transition Piece Static Seal Material Upgrade
Four different types of base materials (NIT50, NIT60, SS304, and SS316) were proposed as a replacement for the originally installed gas turbine transition piece static seal. Samples from each of the proposed material, as well as the original static seal were investigated throughout this study. The specimens were metallographically prepared and heated at three different temperatures (500 oC, 600 oC and 850 oC) for 48 hours. Then examined to investigate the oxidation layer thickness on the different materials at the above-mentioned temperatures simulating the static seals in service conditions. The investigation concluded that, the NIT 50 showed better oxidation resistance at 850 °C compared to other investigated materials.
Keywords:Gas Turbine; Transition Piece, Static Seal
The performance and durability of an industrial gas turbine depends to large extent on the capability of the turbine hot section components to survive the effects of the hot gas stream by resisting surface attack and maintaining their mechanical and physical properties. Turbine hot section components are subjected to very complex and demanding service environments as a result, these components can degrade by a variety of mechanisms. High temperature oxidation and hot corrosion are two of the main degradation mechanisms that occur in industrial gas turbine hot sections. The transition piece static seal function is to prevent leakage of the hot gases out of the gas stream into the combustion casing cavity, which might result in reduction of power and thermal energy of the engine. The original design of the transition piece static seal is made of carbon steel which has poor oxidation and heat resistance properties.
Superalloys based on nickel represent a class of materials intended for applications at high temperatures such as hot-gas-path turbine components. Nickel-based superalloy is the most used material in turbine engines because of its high strength and long fatigue life combined with good resistance to oxidation and corrosion at high temperature. Nickel-based superalloy is the material of choice for the hottest engine components that are required to operate above 800 °C. One of the most remarkable properties of nickel superalloys that justify its utilization in gas turbine engines is their outstanding resistance against creep and stress rupture at high temperature. Creep is an important material property in order to avoid seizure and failure of engine parts. Most materials experience rapid creep at temperatures of 30–40% of their melting point temperature. Nickel superalloys resist creep so well they can be used at 850 °C, which is over 70% of their melting temperature (Tm = 1280 °C). The exceptional creep and stress rupture resistance of nickel superalloys mean that engines can operate at higher temperatures [1]. The microstructure of bimodal steel comprises of fine martensite (≈200–400 nm) in a matrix of coarse austenitic grains (≈10 μm). The bimodal steel shows significantly lower corrosion rate of 0.001 mm/year in 3.5 wt% NaCl solution compared to 0.088 mm/year for commercially available SS316L stainless steel. The exceptional corrosion behaviour of bimodal steel is attributed to favourable electronic properties of its passive layer. Formation of a depletion zone in the passive layer and higher chromium fraction limits the charge transport across metal‐oxide‐solution interface[2].
The objective of this study is to investigate and compare the formed oxidation layer thickness on the new proposed base materials (304SS, 316SS, NIT50 and NIT60) and the originally installed static seal samples at different temperatures (500 °C, 600 °C and 850 °C) for exposure time of 48 hours. Also, recommend the best material for extending the life of transition piece static seal to match the operational life of the rest of the hot gas parts.
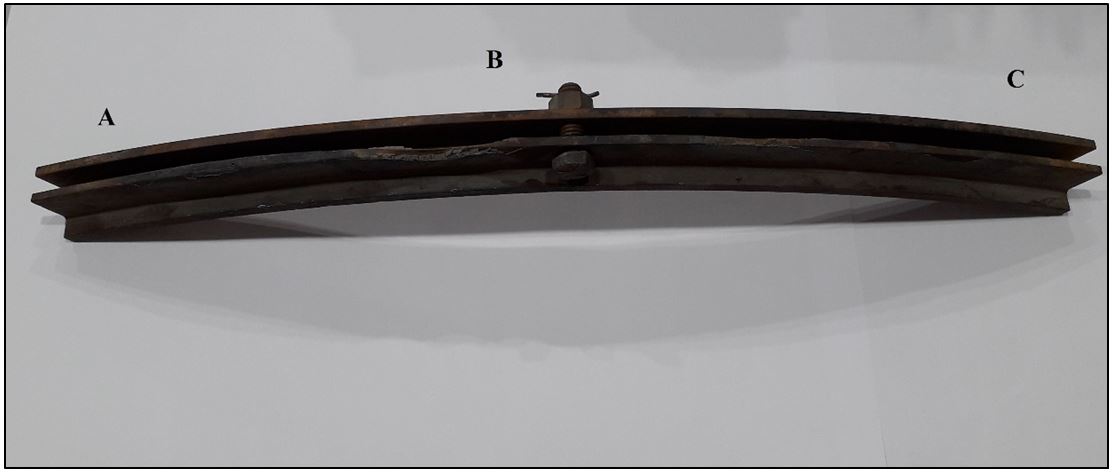
Three test specimens were prepared from the original static seal (locations A, B&C) for spectroscopy examination. The test specimens were sectioned using diamond saw machine Struers-Secotom-10 with speed of 800 rpm, feed rate of 0.15 mm/sec and water-cooling solution. Then the specimens were mounted using compression mounting using epoxy compression mounting resins. Grinding was conducted using SiC grit paper 180,240,300 and 600, followed by polishing using Al3O2 slurry followed by etching using Nital (2% HNO3, 98% ethanol). The specimens were examined in the Quanta ESEM-FEG using the procedure outlined in the ISO-17025 certified SALAM 530-03 method. The ESEM was operated in high vacuum mode at 25kV acceleration potential and 11 mm working distance. Backscattered electron image along with energy dispersive X-ray spectra (EDS) were acquired from the sample. The identity of the elements in the EDS spectra was confirmed by holographic peak deconvolution (HPD). Backscattered electron images along with EDS spectra were acquired from different parts of the examined sample (A, B & C) and the average values were calculated to reduce the error in the analysis (Figure 1) (Table 1).
Four different material types (NIT50, NIT60, SS304, and SS316) were proposed as a base material replacement for the original static seal material. Six samples were collected from each of the proposed replacement materials in addition to three specimens of original static seal were studied. Specimens of each base material were metallographically prepared and subjected to a heat treatment step at different temperatures. The sample were heat treated to temperatures of 500 °C, 600 °C and 850 °C. Samples from different material were isothermally oxidized at the above-mentioned temperatures for 48 hours. In addition, samples from the static seal were oxidized at the same temperatures and time. All the samples were examined in order to observe the formation of the oxide layers at different temperatures and measure their thicknesses.
All samples for either the original static seal base material or the proposed alternative base materials have been exposed to heat treatment at different temperatures of 500 °C, 600 °C and 850 °C for 48 hours. The formation of the oxide-rich layers (Table 2) were observed on the oxidized samples. All samples results are discussed below,
It was observed that the formed oxide layer starts to thicken during oxidation above 500 oC. This formed layer can be ignored at temperature of 500 oC since its thickness is minor with average of about 24 micron (Figure 2). On the other hand, the formation of the oxide layer for the specimen that was exposed to 600 °C accumulated irregularly with different thicknesses at different locations with the average of 177.
Figure 2 (Bottom), also shows the formed oxide layer at magnification of 10X at 850 oC for exposure time of 48 hours. The average thickness of the layer is about 400 micron. In Comparison, the 850 oC formed oxide layer was greater than the formed layer at 500 oC by sixteen times. The oxide layer formed on static seal material is exponentially increased as temperature increases and this result indicates that failure would occur within a short duration of operation at a temperature of 850 °C.
For NIT 50 at 850 °C shown in figure 3 (Top), a negligible very thin layer was accumulated at an average thickness of about 3 micron. On the other hand, NIT 60 at the same temperature in figure 3 (Bottom) showed thicker layer at same magnification by three times comparing to NIT 50 (Figure 3).
Figure 4 (Top & Bottom), shows that the SS304 and SS316 materials have the same behavior at 850 °C with average thickness of 30 microns. The formed oxide layer on SS304 did not accumulated uniformly as well as in the case of SS316.
The investigation showed that the originally installed static seal samples oxidized at a faster rate as opposed to the other examined base materials, thus forming a much thicker oxide layer. The faster rate of oxidation leads to internal stresses within oxide surface layers which tend to amplify with increasing layer thickness causing cracking and spallation, which may compromise the integrity of the static seal during operation.
The investigation showed that there is a formation of oxides-rich layer on the surface of all the heat-treated samples, with the oxide layer formed on the proposed base materials showing different thicknesses.
The average thickness of the formed oxide layer at the original static at 850 °C is 400 micron, while the formed oxide at 500 °C have an average 24 micron, which is sixteen times greater.
NIT 60 at 850 °C, showed thicker oxide layer at magnification of 50X by three times comparing to NIT 50 however, SS316 has a thicker formation layer at 850 °C greater than NIT 60 by at least three times.
NIT 50 shows a better oxidation resistance behavior at 850 °C compared to other proposed materials. The oxidation formed layer could not be seen at 10X magnification but it can be visible at 50X magnification. This result indicates the NIT 50 material is most suitable to replace the current static seal material due to the good oxidation resistance behavior that has been noticed during this study.
To increase component life, the seal should be protected by coating. The fundamental role of high temperature coatings is to delay the onset of substrate deterioration and the attendant effects on component structural integrity.

.JPG)
.JPG)
.JPG)
.JPG)
.JPG)
.JPG)