Concepts of Process Validation in Solid Dosage Form [Tablet] – An Overview
2Department of Pharmaceutics, Al Shifa College of Pharmacy, Kerala, India
3Department of Pharmaceutical Chemistry, School of Pharmacy, International Medical University, Malaysia
Quality is always an imperative prerequisite when considered any product. Therefore, drugs must be manufactured to achieve a predictable therapeutic response to a drug included in a formulation which is capable of large scale manufacture with reproducible and highest quality levels in the finished product. Nowadays validation has become one of the pharmaceutical industry's most recognized, discussed subjects and fond in number of pharmaceutical industries. It is a critical success factor in product approval to get commercialization of the product. Among the various reasons for process validation, the first and primary reason is a regulatory requirement for almost every process in the global health care industry like pharmaceuticals, biologics, and medical devices. Regulatory bodies in the various countries across the world expect that the organization needs to validate their manufacturing processes. Once a process/product is systematically validated, it could be easily justified for the reduced sample size and intervals, which provide a measurable return on the effort of validation. Aside from utility systems, this is hardly ever realized and represents one of the major failings relative to the implementation of validation in pharmaceutical industry [1-3]. Nowadays more sophisticated drug delivery systems are introduced into the market, but tablets are still by far the most widespread solid dosage form in the global world.
Validation is a scientific study of a quality assurance which provides confirmation of the facilities, systems, equipments, manufacturing processes, software and testing methods that have an impact on product quality, safety and efficacy. The outcome of validation studies are
➢ To prove that equipment, system and process is consistently doing what it is supposed to do. (i. e. the process is under control).
➢ To determine the process variables and acceptable limits for these variables and to set up appropriate process control [4-6].
Process validation is defined as "establishing documented evidence which provides a high degree of assurance that a specific system, related equipment and process consistently meet the approved specifications and produce products meeting predetermined quality attributes [7].
The validation activities are performed in accordance with pre-approved written protocols. The facility, utilities, major manufacturing equipment and laboratory instruments should be qualified by performing Design Qualification (DQ)/ Installation Qualification (IQ)/Operational Qualification (OQ)/Performance Qualification (PQ) as per the approved protocols. Protocol is either be designed by the user department or supplied by the vendor. Preferably the skilled personnel engaged internally or externally conduct equipment / instrument qualification.
Design Qualification (DQ): The DQ is aimed to specify that the equipment, system or facility is designed in accordance with the requirements of the user and Good Manufacturing Practice (GMP) guidelines. A protocol should be made for design requirements/technical specifications with consultation of the supplier and a report is documented for the same.
Installation Qualification (IQ): Upon arrival of the equipment in the plant, it is first checked to ensure that the equipment is supplied as per the design requirements/technical specifications. The Engineering Department verify that the equipment and components are supplied in accordance with the specifications mentioned in (DQ). The equipment is then shifted to its predetermined location and place as per the equipment floor Plan. The IQ procedure comprises the following activities. The activities are
Confirmation to the parameter specified in DQ.
1. Equipment description and identification.
2. Verification of major components.
3. Material of construction.
4. Utilities connections.
5. Calibration Requirements
6. Installation verification
7. IQ certification
Installation Qualification is considered completed only after the equipment has been properly installed; all the above said parameters are verified and documented as per the approved IQ protocol. The validation team check, identify and enlist the appropriate safety features provided for various service lines and prepare a preventive maintenance programme.
Operational Qualification (OQ): During Operational Qualification documented evidence are made to establish that all parts of the equipment work within their specifications and operational parameters.
The OQ procedure typically includes the following:
1. Brief identification information
2. Visual inspection parameters
3. Functioning of switches and indicator lights
4. Check and calibration of sensor, probes, gauges, recorders, air flow rates, directions, pressures, temperatures etc.
5. Filter integrity and efficiency test
6. Cleaning procedure
7. Control panel testing
8. Safety features testing
9. Operational testing as per process and system requirements and challenging.
10. Training for operators and supervisors for operation and cleaning of equipment.
The equipment is operated for each operating component to verify that all operating features are functioning as per the specifications. A special attention is paid to service line connections and safety features. Critical devices are identified and calibrated against a standard device.
The completion of a successful operational qualification allow the finalization of calibration, operating and cleaning procedures, operator training and preventive requirements. It shall permit the formal "release" of the facilities, systems and equipments.
Performance Qualification (PQ): Performance qualification is the final stage of qualification, which demonstrates that how the equipment/system will perform when challenged under simulated or actual production conditions. A series of tests are designed to demonstrate that the equipment / system is capable to perform consistently and meet required specifications under routine production operations.
Process validation is defined as "establishing documented evidence which provides a high degree of assurance that a specific system, related equipment and process consistently meet the approved specifications and produce products meeting predetermined quality attributes.
Process validation: Process validation is a basic factor for drug product safety and quality and thus a fundamental component of the quality assurance system used by pharmaceutical manufacturers. The basic principle of Quality Assurance is that a drug should be produced that is fit for its intended use. Effective Process Validation contributes significantly to assure the drug quality; this principle incorporates the understanding that the following conditions exist:
➢ Quality, safety, and efficacy are designed or built into the product.
➢ Quality cannot be adequately assured merely by in-process and finished-product inspection or testing.
➢ Each step of a manufacturing process is controlled to assure that the finished product meets all design characteristics and quality attributes including specifications.
Types of Process validation [11-17]
1. Prospective validation
2. Concurrent validation
3. Retrospective validation
4. Revalidation
Prospective validation: Prospective validation is carried out during the development stage of a product and it is required for new manufacturing formulae or methods of preparation where the latter are adopted. The purpose is to ensure that the defined process, using the materials and equipment specified, should be shown to yield a product that is consistently of the required quality and quantity what it is proposed to do based on the preplanned protocols. In this phase the extent to which deviations from the chosen processing parameters can influence in the product quality is also be evaluated. In general the final batch size should not be more than 10 times the batch size of the representative development batches. The process should include identification and evaluation of individual steps, identification of critical situations, design of trial plans and set of priorities, performance of trials, recording of results, assessment and evaluation of observed results. If the results are unsatisfactory then the processes are modified and improved until acceptable results are obtained. This is essential to limit the risk and errors that may occur on production scale.
Retrospective validation: Retrospective validation is based on a review of historical manufacturing and testing data, and the analysis of accumulated results from past production to assess the consistency of a process. It is assumed that the composition, procedures and equipment remained unchanged.
During retrospective validation results of in-process and final control tests are evaluated. A total of 10-25 batches (or more), manufactured over a period of 12 months, is used for reviewing the results, to provide a statistically significant picture. Quality control charts could be used when performing retrospective validation. Failure investigations should however, be performed separately. All difficulties and failures recorded are analyzed to determine limits of process parameters and product-related problems. These should include rejections, complaints and returns. As retrospective validation is not considered to be a quality assurance measure it should not be applied to new processes or products.
Steps during retrospective validation includes
➢ Choosing a critical quality parameter (e.g. assay value, unit dose uniformity, disintegration time and dissolution).
➢ Extracting the analytical results from each batch (the results of a batch are grouped as subgroups).
➢ Pooling the results from the batches.
➢ Calculating the grand average (process average) and control limits (upper and lower control limits).
➢ Plotting the results on graphs or charts.
Concurrent validation: Concurrent validation is carried out during normal production. This method of validation can only be successful if the development stage has resulted in a proper understanding of the fundamentals of the process. It is carried out during normal production of products intended for sale. It should involve close and intensive monitoring of the steps and critical points for at least first three production scale batches. The in-process control results are used to provide some of the evidence required for validation but these are no substitute for validation. Validation in the production unit mainly comprises of the determination and evaluation of the process parameters of the facilities applied for the scale-up to final batch size. The control of all critical process parameters, the results of the in-process controls, final controls and stability tests should prove the suitability of the important individual steps of a procedure.
Revalidation: In general Revalidation is exploratory review the current performance of the validation effect to confirm the validated status of the facilities, systems, equipments, manufacturing processes, software and testing.
Reasons for performing the revalidation process includes
➢ Transfer of a product from one plant to another plant.
➢ Changes made in the manufacturing process, the cleaning process or other changes which might have impact in the quality of the product.
➢ Systematic periodic checking of the validation results
➢ Change in the batch size.
➢ Consecutive fail in the batch to meet the predetermined specifications with respect to process and product.
➢ Revalidation procedures depend on the extent of the changes made in the process and the effect upon the product.
Order of Priority in Process Validation [18]: It is not possible to validate the company's entire product; hence it is worthy to enlist the various categories of the product to be validated. Higher priority is given to company's most profitable products.
Priority with respect to product the process validation is suggested as follows
A. Sterile Products and Their Processes
1. Large-volume parenterals (LVPs)
2. Small-volume parenterals (SVPs)
3. Ophthalmics, other sterile products, and medical devices.
B. Nonsterile Products and Their Processes
1. Low-dose/high-potency tablets and capsules/transdermal delivery systems (TDDs)
2. Drugs with stability problems
3. Other tablets and capsules
4. Oral liquids, topicals, and diagnostic aids
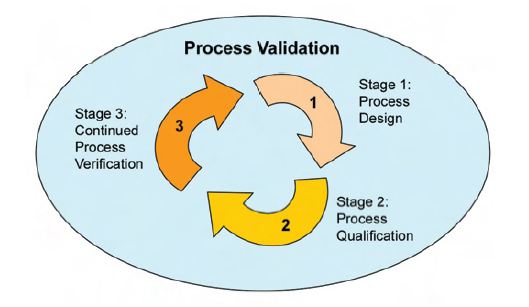
Process Qualification: This stage has two elements: (1) design of the facility and qualification of the equipment and utilities and (2) process performance qualification (PPQ).
Continued Process Verification: The goal of the third validation stage is continual assurance that the process remains in a state of control (the validated state) during commercial manufacture.
This lifecycle approach emphasizes the importance among the three stages are [19-22]
1. Product/process design and development
2. Qualification of the commercial manufacturing equipment and its process
3. Maintenance of the process in a state of control during routine commercial production.
Process validation lifecycle approach between the three stages is shown in Figure 1.
Outline of lifecycle approaches in process validation are
➢ Gain a high degree of assurance in the performance of the manufacturing process by a manufacturer before any batch from the process is commercially distributed for use by consumers.
➢ This assurance is obtained from objective information and data from laboratory, pilot, and/or commercial scale studies – this implies a need for greater scrutiny of process performance during the early stages of commercial manufacture.
➢ A successful validation program depends upon the skilled interpretation of the information and knowledge gained from product and process development regarding sources of variation, its impacts, and the associated risks.
➢ This knowledge and understanding is cited as the basis for establishing the appropriate control strategy during manufacturing process.
➢ Design and development information in the product and process are used to develop the approach in process validation.
➢Testing (in-process, release, characterization) of each significant step of the commercial manufacture process ensure the scientific knowledge of the process.
➢ The significant emphasis in the lifecycle is on maintaining the process in a state of control over the life of the process, which will require ongoing data analysis of both intra-batch and inter-batch variability, and appropriate provisions to address deviations and nonconforming data.
➢ Process validation emphasizes the importance of continued process verification by Quality Assurance (QA) professionals and line operators.
➢ Not surprisingly, the guidance focuses on the importance of demonstrating, documenting, and utilizing process understanding in designing effective validation programs. It provides a strong lead in acknowledging that qualification programs devoid of process understanding will not guarantee the assurance of quality required.
Process validation program can be made more effective and efficient through
➢ Enhanced project management
➢ Scientific knowledge collection, management, and archiving
➢ Collection and assessment of information methods
➢ Integrated team approach
➢ Appropriately documented Project Plans
➢ Involvement of senior management
➢ Statistical assessment of data
Typical pharmaceutical manufacturing processes comprise a serious of unit operations which includes: machinery, methods, people, material, measuring systems and environmental conditions, etc.
Key aspects to be emphasize for the manufacturer are
➢ Understand the process variations
➢ Detect these process variations and assess their extent
➢ Understand the influence on the process and the product
➢ Control such variations on the risk they represent.
To assure batch uniformity and integrity of drug products, written procedures need to be established and followed to test for each batch. Such control procedures are established to monitor the output and to validate the performance of the manufacturing processes that may be responsible for causing variability in the characteristics of in-process material and the drug product.
Modern methods of process control in process validation are [18]
➢ Six sigma
➢ Process capability index
➢ Statistical process control
Statistical process control: Statistical process control (SPC) include
• Sampling plan, experimental design, variation reduction, process capability analysis, process improvement plans
• SPC will not improve a poorly designed product's reliability, but can be used as a tool to maintain the consistency of how the product is made.
In-process specifications establishment: In process specifications are established based on the previous acceptable process average and process variability determined by the application statistical procedures wherever appropriate.
Samples must represent the batch under analysis. Statistical quality control criteria as condition of approval and release of batch must meet its predetermined specifications.
Sampling and testing of in-process materials and drug products
1. Tablet or capsule weight variation
2. Disintegration time
3. Adequacy of mixing to assure uniformity and homogeneity
4. Dissolution time and rate
5. Clarity, completeness or pH of solution.
First four items are related to process validation of solid dosage form.
Items 1 and 3 are associate with variability in the manufacturing process.
Items 2 and 4 are influenced by the ingredient selection in the product formulation.
Item 3 content uniformity and unit potency control is directly related with mixing process.
Formulation Development
• Laboratory function
Process development
• Pilot design
• Design and optimize manufacturing process
• Establish process capability information
Pharmaceutical Manufacturing
• Operate and maintain plant
Engineering
• Installation, quality and certify plant, facilities, equipment and support system.
Quality assurance
• Establish approvable validation protocols and conduct process validation by monitoring, sampling, testing, challenging and auditing the specific manufacturing process>
Analytical Method development or Quality Control
• Laboratory function
Regulatory affairs
• Technical operation representative
IT operation
• Information technology
Important issue to be considered in the process development are:
• Develop the method considering
❖ Laboratory conditions
❖ Quality control settings
• Method chemist must be able to make the method work when
❖ Operating conditions of time
❖ Instruments limitation
❖ Chemist techniques
Process Development Flow:
➢ Process design
• Process flow diagram
• Prepare influence matrix
• Establish experimental procedure
• Establish design criteria
• Prepare study plan an protocols
➢ Challenging Process parameters
• Identify critical variables for unit and overall operation
• Establish maximum tolerance for process variables
➢ Process characterization
• Modify study plan and protocol
• Establish nominal values for critical variables
• Establish tolerance for critical variables
➢ Process verification
• Modify study plan and protocol
• Determine product variability under constant processing conditions
• Prepare process transfer documents
• Finalize product specification
➢ Active pharmaceutical ingredient
➢ Excipients
➢ Variation in raw material is one of the major causes of product variation or deviation from specification
➢ API may represent the most uncontrollable component.
➢ State a good pre-formulation program at early phase of product
➢ Critical steps in the development cycle
• Chemical characteristics
➢ Drug impurities
➢ Impurity levels
➢ Physical properties : Drug morphology, solubility, particle size/surface area, shape, drug density, hygroscopic nature
➢ Accuracy
➢ Precision
➢ Specificity
➢ Intra / Inter day variance
➢ Between operator variation
➢ Between instrument variation
➢ Process equipment
➢ Alternative equipment
➢ Commssioning
➢ Facility qualification
➢ Validation protocol (Qualification)
• IQ
• OQ
• PQ
➢ Process variables
• Variables that control production
❖ Numerical range for each parameters
❖ Establish specification limits
;❖ Prove the process at the extremes of the specification limits
❖ Certify the equipment used to obtain the data and control the process
➢ Mixing or blending
• Wet granulation
❖ Prior granulation to have a uniform drug/excipients
❖ After milling the dried granulation to add other excipients
• Factors in creating a uniform mix or blend
❖ Bulk density
❖ Particle shape
❖ Particle size distribution
❖ Surface area
➢ Items to be consider
• Mixing or blending techniques
❖ Diffusion (tumble)
❖ Convection
❖ Pneumatic (fluid bed)
• Drug uniformity
• Excipient uniformity
❖ Lubricant
❖ Color
❖ Equipment capacity/load
➢ Wet granulation
• Binder addition
• Binder concentration
• Amount of binder solution/granulation solvent
• Binder solution/granulation solvent addition rate
• Mixing time
• Granulation end point
➢ Wet milling
• Equipment size and capacity
• Screen size
• Mill speed
• Feed rate
➢ Milling
• Mill type
• Screen size
• Mill speed
• Feed rate
➢ Drying
• Inlet/outlet temperature
• Airflow
• Moisture uniformity
• Equipment capability/capacity
➢ Tablet compression
• Tooling
❖ Shape
❖ Size
❖ Concavity
• Compression speed
• Compression/ejection force
• In-process test
❖ Appearance
❖ Hardness
❖ Tablet weight
❖ Friability
❖ Disintegration
❖ Weight uniformity
➢ Tablet coating
• Coating purpose
❖ Taste masking
❖ Stability
❖ Controlled release
❖ Product idenification
❖ Aesthetics
❖ Safety material handling
• Tablet properties
❖ Hardness
❖ Shape
• Equipment type
• Coater load
• Pan speed
• Spray guns
• Application/spray rate
• Tablet flow
• Inter/outlet temperature flow
• Coating solution
• Coating weight
• Residual solvent level
• Moisture content of dried granulation
• Granulation particle size distribution affects
❖ Tablet compressibility
❖ Hardness
❖Thickness
❖ Disintegration
❖ Dissolution
❖ Weight variation
❖ Content uniformity
• Blend Uniformity
• Individual tablet/capsule weight
• Tablet hardness
• Tablet thickness
• Disintegration
• Appearance
❖ Tablet mottling
❖ Picking of the monogram
❖ Tablet filming
❖ Capping of the tablets
❖ Tablet colored
• Assay
• Content uniformity
❖ Beginning
❖ Middle
❖ End
• Tablet hardness
• Tablet friability
• Dissolution
During the early stages of process development, parameter target value and tolerance limits are based on good scientific rationale and experience knowledge gained from the earlier and pilot scale studies.
During product and process development both the inputs and outputs of the process are studied. The purpose of the study is to determine the critical parameters and attributes for the process, the tolerance for those parameters and how best to control the various experimental and analytical techniques used for the process characterization.
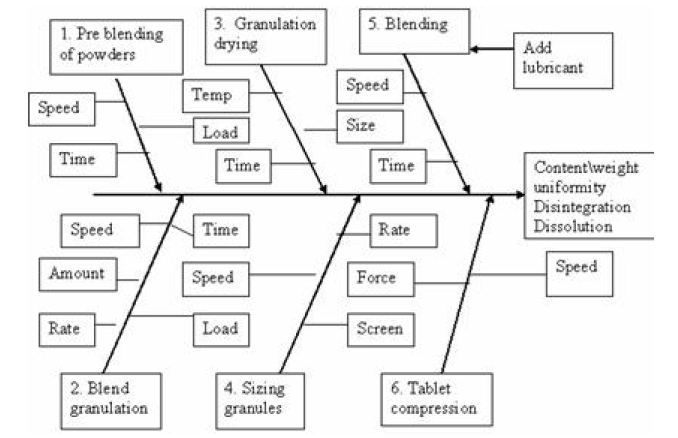
In subsequent product development the parameters and attribute of the process are characteristics to determine the critical parameter of the process, the tolerance limit of the process, and how best to control them. Controllable parameters may be parameters that are adjustable like drying time and temperature. At other time it may be desirable to fix a parameter by specifically setting one value and not testing around the variability. A cause and effect relationship may be established for parameters and desired attributes. Critical quality attributes are dissolution, assay, blend and tablet uniformity and stability. A cause and effect relationship for a solid dosage form is shown in Figure 2.
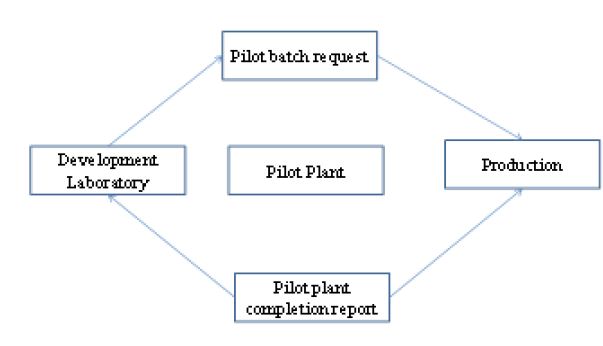
The pilot production phase is carried out either as a shared responsibility between the development laboratories and its appropriate manufacturing counterpart. The joint pilot operation between the development laboratory and production is shown in Figure 3.
Scale up studies
➢ Its process leaving the development laboratory
➢ Critical parameters should be determined by scientific judgment and typically should be based on knowledge derived from research or manufacturing experiences
➢ Prior to its acceptance by full scale manufacturing unit
Scale up activities
➢ Formulation design, selection and optimization
➢ Preparation of the first pilot laboratory batch
➢ Conduct initial accelerated stability testing
➢ If formulation is considered to be stable, additional pilot laboratory batches in order to expand non-clinical and clinical use.
Laboratory Batch
First step in scale up process
➢ Initial design criteria, requirements, and specification
➢ Selection preliminary formula
➢ Scale 1x(3-10kg) for solid
➢ 3000 to 10000 units tablets or capsules
Laboratory Pilot Batch
➢ 10 x (30-100 kg)
➢ 30,000 to 100,000 units
➢ Small pilot equipment within a designated cGMP approved area
➢ Size of the batch depend upon
• Equipment availability
• API
• Cost of raw material
• Inventory requirements for clinical and nonclinical studies
Pilot Production
➢ 100 x 300-1,000 kg
➢ 300,000 – 1,000,000 dosage form units
Batch size of a product
Batch size of a product depends upon the availability of
Equipment
Raw material
Blending: Blend time, rotation rate, agitator speed, room temperature, humidity.
Dry granulation (Roller compaction): Roll speed, feed screw speeds, roll force/pressure, roll separation/gap, room temperature/humidity
Milling: Impeller speed, feed rate, room temp, humidity.
Fluid bed granulation: Granulation fluid mixing time, fluid mixing speed, fluid amount, fluid addition rate, fluid temperature, spray nozzle air volume, bed mixing time, supply air flow rate, dew point, product bed temperature, exhaust air temperature, filter shaking intervals.
Wet granulation: Granulation fluid mixing time, fluid mixing speed, fluid amount, fluid addition rate, fluid temperature, spray nozzle air volume, drug and wet mixing time, impeller speed, chopper speed, power consumption.
Cabinet drying: Supply air temperature, drying time, final moisture content
Fluid bed drier: Supply air flow rate, temperature, product bed temperature, exhaust air temperature, filter shaking intervals, final moisture content.
Compression: Tablet weight, turrent speed, main compression force, pre compression force, feeder speed, upper punch entry, room temperature, humidity.
Coating: Coating suspension mixing time, mixing speed, amount, spray rate, atomization, pressure, pan rotation speed, pre heat time, supply air flow rate, temperature, product bed temperature, exhaust air temperature.
As R&D has established the desired operating range of parameters and attributes, manufacturing should monitor both the parameters and attributes over time and review the information at a pre determined frequency with emphasis on critical key parameters by trend chart, control chart, and process capability. Process specific improvement by flow charts, fish bone diagram, QFD, FMEA, pareto chart, DOE (Design of experiments), correlation analysis, F-test, Scatter diagrams.
Materials, instrumentation, machine calibration and maintenance, human factors and plant layout. The Plant layout includes environmental factory like temperature, pressure, humidity, general cleanliness, orderliness and layout of an area provides an indirect effect on the variation of a product.
Nowadays Validation is the art of designing and practicing the designed steps together with the documentation in pharmaceutical industry. Validation itself does not improve processes but confirms that the processes have been properly developed and are under control in achieving, maintaining the quality of the final product. Application of validation principles will ensure to maintain quality, consistency and reproducibility in product manufacturing process and safety of the pharmaceutical products required from regulatory agencies across the world. The multidisciplinary validation team must identify, study the product and process characteristics and incorporate the important required validation key parameters to ensure that that product will meet all quality, manufacturing, and regulatory requirements. Process validation in solid dosage form is a systematic approach in identifying, measuring, evaluating, documenting and re-evaluating the critical steps in the pharmaceutical solid dosage form manufacturing process with control to assure consistency in the quality of final product. From this review we can conclude that the pharmaceutical process validation and process controls are important steps in manufacturing of solid dosage form with consistent to meet the regulatory required standard such as identity, strength, quality, purity and stability in the final solid dosage form [tablet].
- Agalloco J, Carleton FJ (2008) Validation of Pharmaceutical Processes (3rd edn), Informa Healthcare, New York, USA.
- Tarun Virmani (2007) Validation: An Essentiality In The Pharmacy.
- Patel RC, Bhuva CK, Singh RP, Abhishek D, Sharma A (2011) Pharmaceutical Process Validation Why to Do, When to Do and How to Do it.
- Centre for drug evaluation and research, FDA (2008) Guideline on general principles of process validation.
- Dietrick JM, Loftus BT (2003) The regulatory basis for Process Validation: Pharmaceutical process validation. (3rd edn), Marcel Deckker, NY, USA. 1-8.
- Saraf S, Dashora K, Singh D, Swarnlata Saraf (2005) Validation - the Essential Quality Assurance Tool for Pharma Industries. 3: 45-7.
- Lambert J (2004) Validation Guidelines For Pharmaceutical Dosage Forms. Health Canada / Health Products and Food Branch Inspectorate. 7-15.
- Peither TL (2003) Equipment and facility qualification Pharmaceutical process validation (3rd edn), Marcel Deckker, NY, USA 443-63.
- Chitlange SS, Pawar AS, Pawar HI, Bhujbal SS, Kulkarni A (2006) A Validation.
- Guidance for Industry Process Validation (2011) General Principles and Practices
- Chao AY, John Forbes F (2003) Prospective process validation Pharmaceutical process validation. (3rd edn), Marcel Deckker, NY, USA.
- Trubinski CJ (2003) Retrospective validation: Pharmaceutical process validation. (3rd edn), Marcel Deckker, NY, USA.
- Ahir KB, Singh KD, Yadav SP, Patel HS, Poyahari CB (2014) Overview of Validation and Basic Concepts of Process Validation. Scholars Academic Journal of Pharmacy 3: 178-90.
- Parashar B, Gupta R, Khurana G (2013) A review on process validation as an essential Process. International Journal of Research in Pharmaceutical Sciences 4: 226-9.
- Chaitanya Kumar G, Rout RP, Ramtake S, Bhatacharya S (2005) Process Validation. The Indian Pharmacist 14-9.
- Kathiresan K, Kiran K (2005) Basis of validation- Pharmaceutical Prospective. (1st edn), K.K. Publishers, Chidambaram 32-46.
- Varshney P, Shah M, Patel P, Rohit M (2013) Different aspects involved in process validation. Innovare Journal of Science 1: 16-9.
- Nash RA, Wachter AH (2003) Introduction: Pharmaceutical process validation. (3rd edn), Marcel Deckker, New York, USA.
- Calnan N, Redmond A, O'neill S (2009) The FDA's draft process validation guidance – A perspective from industry. Pharmaceutical Engineering 10-6.
- International Conference on Harmonisation (ICH) (2009) Guideline for industry. Q8: Pharmaceutical Development.
- International Conference on Harmonisation (ICH) (2005) Guideline for industry. Q9: Quality Risk Management.
- International Conference on Harmonisation (ICH) (2005) Guideline for industry. Q10: Pharmaceutical Quality Systems.
- Bankar GS, Anderson NR (1987) Tablets: The theory and practice of industrial pharmacy (3rd edn), Varghese publishing house, Mumbai, India.
- Sharp JR (1986) The problems of process validation. Pharmacy Journal 1: 43-5.
- Jatto E, Okhamafe AO (2002) An Overview of Pharmaceutical Validation and Process Controls in Drug Development. Tropical Journal of Pharmaceutical Research 1: 115-22.
- Chow S (1997) Pharmaceutical Validation and Process Controls in Drug Development. Drug Information Journal 31: 1195-201.
- Center for Drug Evaluation and Research, US Food and Drug Administration (1987) Guideline on General Principles of Process Validation. Washington DC, USA.
- WHO (2014) Good Manufacturing Practices for Pharmaceutical Products. Guidelines on Validation of Manufacturing Process, Geneva, Switzerland.
- Gupta S, Saini S, Singh G, Rana AC (2012) Industrial Process validation of tablet dosage form: An overview. International Research Journal of Pharmacy 3: 48-54.
- Glodek M, Liebowitz S, McCarthy R, McNally G, Oksanen C, et al. (2006) Process robustness - A PQRI white paper. Pharmaceutical Engineering 1-11.
- Teja S (2011) Process validation: An essentially in the pharmaceutical Industry. International Journal of Pharmaceutical Research and Development 3: 133-42.
- ISPE (2012) Stage 2 Process Validation: Determining and Justifying the Number of Process Performance Qualification Batches.
- ISPE (2012) Stage 3 Process Validation: Applying Continued Process Verification Expectations to New and Existing Products.
- WHO (2006) Supplementary guidelines on good manufacturing practices: validation. Technical Report Series.
- Ajay S, Seema S (2013) Process Validation of Solid Dosage Form: A Review. International Journal of Research in Pharmacy and Science 3: 12-30.
- Sharma V, Seth N (2014) Industrial process validation of tablet dosage form: a review. International Journal of Pharmacy Review & Research 4: 80-4.
- Rudolph JS, Seplyak RJ (2003) Validation of solid dosage forms: Pharmaceutical process validation. (3rd edn), Marcel Deckker, NY, 159-90.
- Jena S, Arjun G, Ravipati NVAK, Satish kumar D, Vinod KR, et al. (2010) Industrial process validation of solid dosage forms – an overview. International Journal of Pharmaceutical Sciences Review and Research 4: 145-54.
- Aleem H, Zhao Y, Lord S, McCarthy T, Sharratt P (2003) Pharmaceutical process validation: an overview. Journal of Process Mechanical Engineering 217: 141-51.
- Author Information
-
Lakshmana Prabu S1*, Suriyaprakash TNK2, Ruckmani K1 and Thirumurugan R3 1Department of Pharm. Technology, Bharathidasan Institute of Technology, Anna University, Tiruchirappalli, India 2Department of Pharmaceutics, Al Shifa College of Pharmacy, Kerala, India 3Department of Pharmaceutical Chemistry, School of Pharmacy, International Medical University, Malaysia - Article history
-
Received Accepted Published 31 May 2014 20 June 2014 16 August 2014 - View Pdf
-

- Tables and Figures
-
IMAGE 1 IMAGE 2 IMAGE 3 - Citation
-
Lakshmana Prabu S, Suriyaprakash TNK, Ruckmani K, Thirumurugan R (2014) Concepts of Process Validation in Solid Dosage Form [Tablet] - An Overview. SAJ Pharma Pharmacol 1: 103. doi: 10.18875/2375-2262.1.103